ЬтФПФкШн
ЖдПЩФцЗДгІaAЃЈgЃЉЃЋbBЃЈgЃЉ![]() cCЃЈgЃЉЃЋdDЃЈgЃЉДяЕНЦНКтЪБИїЮяжЪЕФ
ХЈЖШТњзуЃК
cCЃЈgЃЉЃЋdDЃЈgЃЉДяЕНЦНКтЪБИїЮяжЪЕФ
ХЈЖШТњзуЃК![]() ЃНKЮЊвЛГЃЪ§ЁЃЦфЗДгІЕФKжЕжЛгыЮТЖШгаЙиЃЌЯжвЛЗДгІCOЃЈgЃЉЃЋH2OЃЈgЃЉ=CO2ЃЈgЃЉЃЋH2ЃЈgЃЉЃЛЁїHЃО0ЃЌдк850ЁцЪБЃЌKЃН1ЁЃ
ЃНKЮЊвЛГЃЪ§ЁЃЦфЗДгІЕФKжЕжЛгыЮТЖШгаЙиЃЌЯжвЛЗДгІCOЃЈgЃЉЃЋH2OЃЈgЃЉ=CO2ЃЈgЃЉЃЋH2ЃЈgЃЉЃЛЁїHЃО0ЃЌдк850ЁцЪБЃЌKЃН1ЁЃ
ЃЈ1ЃЉШєЩ§ЮТЕН950ЁцЃЌДяЕНЦНКтЪБЃЌK________1ЃЈЬюЃМЁЂЃОЛђЃНЃЉЁЃ
ЃЈ2ЃЉ850ЁцЪБЃЌЙЬЖЈШнЛ§УмБеШнЦїжаЃЌЗХШыЛьКЯЮяЃЌЦ№ЪМХЈЖШc(CO)ЃН0.01molЁЄLЃ1ЁЂ cЃЈH2OЃЉЃН0.05molЁЄLЃ1ЁЂcЃЈCO2ЃЉЃН0.01molЁЄLЃ1ЁЂcЃЈH2ЃЉЃН0.01molЁЄLЃ1ЃЌдђЗД гІПЊЪМЪБH2OЕФЯћКФЫйТЪБШЩњГЩЫйТЪ________ЃЈЬюДѓЁЂаЁЁЂВЛФмШЗЖЈЃЉЁЃ
ЃЈ3ЃЉБЃГжЮТЖШЁЂШнЦїЕФЬхЛ§ВЛБфЃЌШєЭљШнЦїжаГфШыЪЪСПЕФH2SЦјЬхЃЌдђжиаТДяЕНЦНКт ЪБЃЌCOЕФЯћКФЫйТЪБШГфH2SжЎЧА________ЃЈЬюдіДѓЁЂМѕаЁЁЂВЛФмШЗЖЈЃЉЃЌРэгЩЪЧ________ЁЃ
НтЮіЃК
ЃЈ1ЃЉЃО ЃЈ2ЃЉДѓ ЃЈ3ЃЉдіДѓ вђЮЊH2S=H2ЃЋSЃЌдіДѓH2ЕФХЈЖШЃЌдЦНКтЯђФцЗДгІЗНЯђвЦЖЏЃЌCOХЈЖШдіДѓЃЌжиаТДяЦНКтЪБЃЌCOЕФЯћКФЫйТЪБШдРДЦНКтЕФДѓ
|

 УПШе10ЗжжгПкЫуаФЫуЫйЫуЬьЬьСЗЯЕСаД№АИ
УПШе10ЗжжгПкЫуаФЫуЫйЫуЬьЬьСЗЯЕСаД№АИ
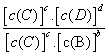
 cC(g)ЃЋdЃФ(g)ДяЕНЦНКтЪБЃЌИїЮяжЪЕФЮяжЪЕФСПХЈЖШТњзувдЯТЙиЯЕЃК
cC(g)ЃЋdЃФ(g)ДяЕНЦНКтЪБЃЌИїЮяжЪЕФЮяжЪЕФСПХЈЖШТњзувдЯТЙиЯЕЃК (ЮЊвЛГЃЪ§)ЃЌKГЦЮЊЛЏбЇЦНКтГЃЪ§ЃЌЫќЕФжЕжЛгыЮТЖШгаЙиЁЃЯжгаЗДгІЃКCO(g)ЃЋH2O(g)
(ЮЊвЛГЃЪ§)ЃЌKГЦЮЊЛЏбЇЦНКтГЃЪ§ЃЌЫќЕФжЕжЛгыЮТЖШгаЙиЁЃЯжгаЗДгІЃКCO(g)ЃЋH2O(g)