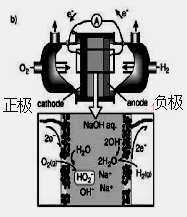
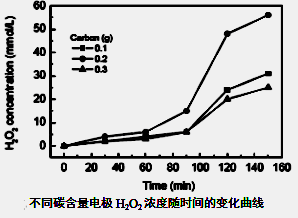
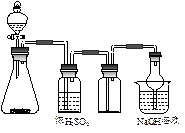��Ŀ����
������ʵ���������ÿ�����þ��Ϊԭ����ȡ��������þ��Mg3N2������֪ʵ���п��ܻᷢ�����з�Ӧ��
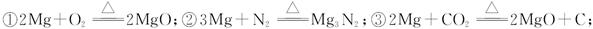

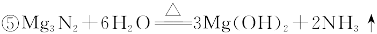
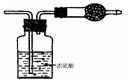
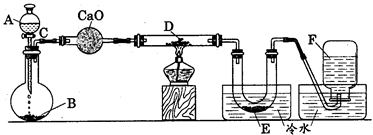
�ɹ�ѡ���װ�ú�ҩƷ����ͼ��ʾ��þ�ۡ���ԭ���۾��Ѹ��װ�����������ķ�Ӧ����ȫ�ģ�����װ�õ�ĩ����������������
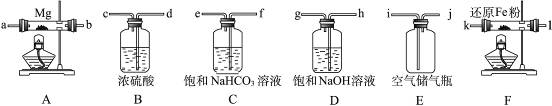
�ش��������⣺
��1�������ʵ�鷽��ʱ����װ��A��E�⣬��Ӧѡ���װ�ã�����ĸ���ţ�����Ŀ�ķֱ���________________________________________________________________________________
_______________________________________________________________________________��
��2�����Ӳ����ʵ��װ�õ������ԡ�ʵ�鿪ʼʱ��������ˮ�Ŀ��أ���������5���Ĵ���ƿѹ�뷴Ӧװ�ã��������������ܵ�˳���ǣ�����ĸ���ţ�______________________________��
��3��ͨ�������ͬʱ��ȼA��Fװ�õľƾ��ƣ���ʵ�����к�Ӱ��? __________��ԭ����_____________________________________________________________________________��
��4�������һ��ʵ�飬��֤�����ǵ���þ��________________________________________
_______________________________________________________________________________��
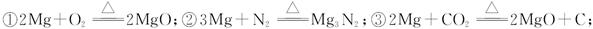

�ɹ�ѡ���װ�ú�ҩƷ����ͼ��ʾ��þ�ۡ���ԭ���۾��Ѹ��װ�����������ķ�Ӧ����ȫ�ģ�����װ�õ�ĩ����������������

�ش��������⣺
��1�������ʵ�鷽��ʱ����װ��A��E�⣬��Ӧѡ���װ�ã�����ĸ���ţ�����Ŀ�ķֱ���________________________________________________________________________________
_______________________________________________________________________________��
��2�����Ӳ����ʵ��װ�õ������ԡ�ʵ�鿪ʼʱ��������ˮ�Ŀ��أ���������5���Ĵ���ƿѹ�뷴Ӧװ�ã��������������ܵ�˳���ǣ�����ĸ���ţ�______________________________��
��3��ͨ�������ͬʱ��ȼA��Fװ�õľƾ��ƣ���ʵ�����к�Ӱ��? __________��ԭ����_____________________________________________________________________________��
��4�������һ��ʵ�飬��֤�����ǵ���þ��________________________________________
_______________________________________________________________________________��
��1��B��Ŀ���dz������е�ˮ���������ⷴӦ�ܷ�����D��Ŀ���dz������е�CO2�����ⷴӦ�۷�����F��Ŀ���dz������е����������ⷴӦ�ٷ���
��2��j��h��g��d��c��k��l����l��k����a��b����b��a��
��3��ʹ����þ���������װ��F�еĻ�ԭ����û�дﵽ��Ӧ�¶�ʱ���������ܳ�������������ͬþ��Ӧ����ʹ����þ�л�������þ
��4��ȡ������������Թ��У��μ�����ˮ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����Թ��е���Һ���ֻ��ǣ���ɫʯ����ֽ�����������֤���е���þ����
��2��j��h��g��d��c��k��l����l��k����a��b����b��a��
��3��ʹ����þ���������װ��F�еĻ�ԭ����û�дﵽ��Ӧ�¶�ʱ���������ܳ�������������ͬþ��Ӧ����ʹ����þ�л�������þ
��4��ȡ������������Թ��У��μ�����ˮ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����Թ��е���Һ���ֻ��ǣ���ɫʯ����ֽ�����������֤���е���þ����
��1����Mg����O2��CO2��H2O������Ӧ��Ӧ��ȥ�����е�H2O��O2��CO2����B����ȥ�����е�ˮ��������D����ȥ�����е�CO2����F����ȥ�����е�O2��
��2����ȥ�����е�H2O��O2��CO2��˳��Ӧ�ȳ�CO2�ٳ�ȥˮ����������ȥO2��
��3�����ͬʱ��ȼA��Fװ�õľƾ��ƣ�A��Ӳ�ʲ������п���û���ž�����ʱMg����H2O��CO2��O2�ȷ�Ӧ������������
��4������Mg3N2��ˮ�ܷ���ˮ�ⷴӦ������Mg��OH��2��NH3����˿�����ʪ��ĺ�ɫʯ����ֽ�������Ƿ����NH3������֤����Mg3N2���ɡ�
��2����ȥ�����е�H2O��O2��CO2��˳��Ӧ�ȳ�CO2�ٳ�ȥˮ����������ȥO2��
��3�����ͬʱ��ȼA��Fװ�õľƾ��ƣ�A��Ӳ�ʲ������п���û���ž�����ʱMg����H2O��CO2��O2�ȷ�Ӧ������������
��4������Mg3N2��ˮ�ܷ���ˮ�ⷴӦ������Mg��OH��2��NH3����˿�����ʪ��ĺ�ɫʯ����ֽ�������Ƿ����NH3������֤����Mg3N2���ɡ�

��ϰ��ϵ�д�
 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�
�����Ŀ
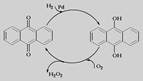

 (NH4)2S2O8 + H2����
(NH4)2S2O8 + H2����