��Ŀ����
��2008?ɽ�������ǵ����ϼ�Ϊ�ḻ��Ԫ�أ�
��1��Li3N�����е���N3-���ڣ���̬N3-�ĵ����Ų�ʽΪ
��2��N��N�ļ���Ϊ942kJ?mol-1��N-N�����ļ���Ϊ247kJ?mol-1������˵��N2�е�
��3����CH3��3NH+��AlCl4-���γ�����Һ�壮����Һ��������������ɣ��۵����100�棬��ӷ���һ����л��ܼ�
a����ȼ�� b������ɫ���ܼ� c�����ϲ��� d�����Ȳ���
��4��X+�����е������ó���K��L��M�������Ӳ㣬����N3-�γɵľ���ṹ��ͼ��ʾ��X��Ԫ�ط�����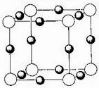
��1��Li3N�����е���N3-���ڣ���̬N3-�ĵ����Ų�ʽΪ
1S22S22P6
1S22S22P6
����2��N��N�ļ���Ϊ942kJ?mol-1��N-N�����ļ���Ϊ247kJ?mol-1������˵��N2�е�
��
��
������
��
���ȶ�������ҡ��С�����3����CH3��3NH+��AlCl4-���γ�����Һ�壮����Һ��������������ɣ��۵����100�棬��ӷ���һ����л��ܼ�
С
С
�����������������b
b
������ţ���a����ȼ�� b������ɫ���ܼ� c�����ϲ��� d�����Ȳ���
��4��X+�����е������ó���K��L��M�������Ӳ㣬����N3-�γɵľ���ṹ��ͼ��ʾ��X��Ԫ�ط�����
Cu
Cu
����ͬһ��N3-������X+��6
6
����
��������1��N3-����ԭ�Ӻ����10�����ӣ������������ԭ����д�������Ų�ʽ��
��2��N��N�к���2���м���1���Ҽ�����֪N��N����Ϊ942kJ/mol��N-N��������Ϊ247kJ/mol����1���м��ļ���Ϊ
kJ/mol=347.5kJ/mol���Դ��жϣ�
��3������Һ���е������������Ӽ�����������ǿ������ͷ��Ӽ����������Դ˽��
��4�������ж�XΪCuԪ�أ����þ�̯�����㣮
��2��N��N�к���2���м���1���Ҽ�����֪N��N����Ϊ942kJ/mol��N-N��������Ϊ247kJ/mol����1���м��ļ���Ϊ
| 942-247 |
| 2 |
��3������Һ���е������������Ӽ�����������ǿ������ͷ��Ӽ����������Դ˽��
��4�������ж�XΪCuԪ�أ����þ�̯�����㣮
����⣺��1��Nԭ��ԭ������Ϊ7��N3-����ԭ�Ӻ����10�����ӣ��������ﵽ�ȶ��ṹ�������������ԭ����д�������Ų�ʽΪ1S22S22P6��
�ʴ�Ϊ��1S22S22P6��
��2��N��N�к���2���м���1���Ҽ�����֪N��N����Ϊ942kJ/mol��N-N��������Ϊ247kJ/mol����1���м��ļ���Ϊ
kJ/mol=347.5kJ/mol����N2�еĦм����ܴ��ڦҼ����ܣ����ȶ����ʴ�Ϊ���У��ң�
��3������Һ���е������������Ӽ�����������ǿ������ͷ��Ӽ���������������ӷ��Ծ�С��������Ⱦ�������ǡ���ɫ���ܼ����ʴ�Ϊ��С��b��
��4��X+��������=2+8+18=28������XΪ29��CuԪ�أ���ͼ�Ͽ����١�࣬���X3N֪����ΪN3-���Զ����ϵġ�Ϊ���ģ����������X+��3��������һ����������ά�ռ���8�������������壬��ÿ��N3-��Χ��X+��8��3��
=6��������ԭ��Ϊ4�����������ã�����ÿ��ԭ���ڴ˾�����Ϊ
�ݣ���
�ʴ�Ϊ��Cu��6��
�ʴ�Ϊ��1S22S22P6��
��2��N��N�к���2���м���1���Ҽ�����֪N��N����Ϊ942kJ/mol��N-N��������Ϊ247kJ/mol����1���м��ļ���Ϊ
| 942-247 |
| 2 |
��3������Һ���е������������Ӽ�����������ǿ������ͷ��Ӽ���������������ӷ��Ծ�С��������Ⱦ�������ǡ���ɫ���ܼ����ʴ�Ϊ��С��b��
��4��X+��������=2+8+18=28������XΪ29��CuԪ�أ���ͼ�Ͽ����١�࣬���X3N֪����ΪN3-���Զ����ϵġ�Ϊ���ģ����������X+��3��������һ����������ά�ռ���8�������������壬��ÿ��N3-��Χ��X+��8��3��
| 1 |
| 4 |
| 1 |
| 4 |
�ʴ�Ϊ��Cu��6��
���������⿼�龧���ļ����Լ���ѧ�����������͵�֪ʶ����Ŀ�Ѷ��еȣ��״���Ϊ��3����ע�����Ӽ�������ͷ��Ӽ�������Ҫǿ��

��ϰ��ϵ�д�
�����Ŀ