��Ŀ����
ʯī�ڲ�����������ҪӦ�á�ij����ʯī�к�SiO2��7.8%����Al2O3(5.1%)��Fe2O3(3.1%)��MgO(0.5%)�����ʡ���Ƶ��ᴿ���ۺ�Ӧ���������£�
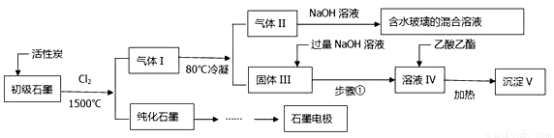
��ע��SiCl4�ķе���57.6ºC�������Ȼ���ķе������150ºC��
��1����֪1molʯī��ȫת��Ϊ���ʯ��Ҫ����1.9kJ����������д��ʯīת��Ϊ���ʯ���Ȼ�ѧ��Ӧ����ʽ�� _________________________��
��2������Ӧ����ͨ��Cl2ǰ����ͨһ��ʱ���N2�����·�Ӧ��ʯī�е����������ʾ�ת��Ϊ��Ӧ�Ȼ��80��������Ŀ���ǣ�_____________________�����ɻ���̿�õ������Ļ�ѧ��Ӧ����ʽΪ��_____________________��
��3���������NaOH��Һ�õ���ҺIV�����ӷ�Ӧ����ʽΪ��_________________________��
��4���û�ѧ��Ӧԭ�����͵õ�����V�Ĺ��������������ͼ��ȵ����ã�________��1kg����ʯī���ɻ��V������Ϊ_______________kg��
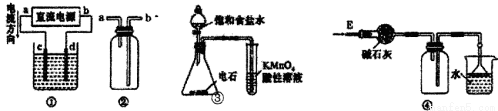
KMnO4��Һ������������ԭ��Ӧ�ζ��ı�Һ������KMnO4��ǿ�����ԣ�������Һ�����ױ������л�ˮ��ijЩ������ԭ�����ʻ�ԭ����������������MnO ��OH��2���������KMnO4����Һ�IJ����ǣ� ��1����ȡ�Զ�����������KMnO4��������ˮ������Һ���Ȳ�������1h�� ��2�����ײ���©�����˳�ȥ���ܵ�MnO ��OH��2�� ��3�����˵õ���KMnO4��Һ��������ɫ�Լ�ƿ���ڰ����� ��4������������ԭ�ζ���������700C-800C�������û��Լ� �����ȸߡ�ʽ���ϴ��ȶ��ԽϺõ����ʣ���Һ�궨��Ũ�ȡ���ش��������⣺
��1������Һ���Ȳ�������1h��Ŀ���� ��
��2����α�֤��700C~800C�����½��еζ������� ��
��3��ȷ��ȡһ�������KMnO4��Һ�� ����������������
��4�������������У����ڱ궨KMnO4��Һ�Ļ��Լ����ѡ�� �����������
A��H2C2O4��2H2O | B��FeSO4 | C��Ũ���� | D��Na2SO3 |
��5����ȷ��ȡWg��ѡ�Ļ��Լ�����ˮ���500mLˮ��Һ��ȡ25��00mL������ƿ�У��ø��������Һ�ζ����յ㣬���ĸ��������ҺVmL���ʣ�
�ٵζ��յ��־�� ��
��������KMnO4����Һ�����ʵ���Ũ��Ϊ ��
��6�����÷������ܵ�KMnO4����Һȥ�ζ�ˮ����Fe2+��������õ�Ũ��ֵ�� ���ƫ�ߡ���ƫ�͡�����
���и���ʵ�������ʵ���������ó��Ľ��ۣ���ȷ����
A | ��2ml2%��CuSO4��Һ�м���0.5ml 1%��NaOH��Һ����μӼ���M��Һ������ | δ����ש��ɫ���� | M�в���ȩ�� |
B | ��CuSO4��Һ�м���KI��Һ���ټ��뱽������ | �а�ɫ�������ɣ�������Ϻ�ɫ | ��ɫ��������ΪCuI |
C | ���л��Լ�N�м���2mL5%��NaOH��Һ�����ȣ���ȴ��ȡ�ϲ���Һ�μӼ���AgNO3��Һ | ���ֺ�ɫ���� | N����±���� |
D | ��Fe(NO3)2��Ʒ����ϡH2SO4�μ�KSCN��Һ | ��Һ��ΪѪ��ɫ | Fe(NO3)2��Ʒ���������� |


 �͡������ͬԪ�ص�ԭ�ӡ�
�͡������ͬԪ�ص�ԭ�ӡ�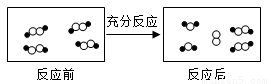

 Ce��(H2n-4A2n)+4H+��ʵ��������ȡʱ�õ�����Ҫ������������Ϊ____________����ͼ��Dʱ����ȣ���ʾCe(��)�ֱ����л�������ˮ���д�����ʽ�����ʵ���Ũ��֮��(
Ce��(H2n-4A2n)+4H+��ʵ��������ȡʱ�õ�����Ҫ������������Ϊ____________����ͼ��Dʱ����ȣ���ʾCe(��)�ֱ����л�������ˮ���д�����ʽ�����ʵ���Ũ��֮��( )�����������������䣬����ʼ��Һ�м��벻ͬ����Na2SO4�Ըı�ˮ���е�c(SO42-)��D����ʼ��Һ��c(SO42-)�仯��ԭ��__________��
)�����������������䣬����ʼ��Һ�м��벻ͬ����Na2SO4�Ըı�ˮ���е�c(SO42-)��D����ʼ��Һ��c(SO42-)�仯��ԭ��__________��