��Ŀ����
����Ŀ����Ϊ��Ҫ����ԭ��,�й㷺��;��
��1���ϳɰ��е������������з�Ӧ��ȡ:
a.CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H=+216.4kJ/mol
CO(g)+3H2(g) ��H=+216.4kJ/mol
b.CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H=-41.2kJ/mol
CO2(g)+H2(g) ��H=-41.2kJ/mol
��ӦCH4(g)+2H2O(g)![]() CO2(g)+4H2(g) ��H=_________��
CO2(g)+4H2(g) ��H=_________��
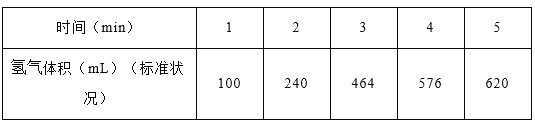
��2����ʼʱͶ�뵪���������ֱ�Ϊ1mol��3mol���ڲ�ͬ�¶Ⱥ�ѹǿ�ºϳɰ���ƽ��ʱ������а�������������¶ȹ�ϵ����ͼ��
�ٺ�ѹʱ,��Ӧһ���ﵽƽ��״̬�ı�־��______(�����)��
A.N2��H2��ת������� B.��Ӧ��ϵ�ܶȱ��ֲ���
C. ![]() ��ֵ���ֲ��� D.
��ֵ���ֲ��� D. ![]() =2
=2
��P1_____P2(�>������<������=��������ȷ��������ͬ)����Ӧƽ�ⳣ��:B��____D�㣻
��C��H2��ת����____����A��B����������,�÷�Ӧ�ӿ�ʼ��ƽ��ʱ���ɰ���ƽ������:v(A)______v(B)��
��3��N2H4��������ƽ�����NH3��NaClO��һ�������·�Ӧ������N2H4��
��д��NH3��NaClO��Ӧ����N2H4�Ļ�ѧ����ʽ__________��
����֪25��ʱN2H4ˮ��Һ��������:N2H4+H2O![]() N2H5++OH- K1=1��10-a��N2H5++H2O
N2H5++OH- K1=1��10-a��N2H5++H2O![]() N2H62++OH- K2=1��10-b��
N2H62++OH- K2=1��10-b��
25��ʱ,��N2H4ˮ��Һ�м���H2SO4,��ʹc(N2H5+)>c(N2H4),ͬʱc(N2H5+)>c(N2H62+),Ӧ������ҺpH��Χ_________(�ú�a��bʽ�ӱ�ʾ)��
���𰸡� +175.2kJ/mol BC < > 66.7% < 2NH3+NaClO![]() N2H4+NaCl+H2O (14-b��14-a)
N2H4+NaCl+H2O (14-b��14-a)
����������1��a+b�ɵ÷�ӦCH4(g)+2H2O(g)![]() CO2(g)+4H2(g)�����ݸ�˹���ɣ���H=+216.4kJ/mol+(-41.2kJ/mol)=+175.2kJ/mol��
CO2(g)+4H2(g)�����ݸ�˹���ɣ���H=+216.4kJ/mol+(-41.2kJ/mol)=+175.2kJ/mol��
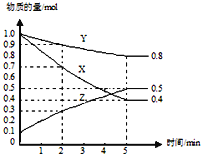
��2����A�������������ʼ���ʵ���֮��Ϊ1:3���ɷ�Ӧ����ʽN2+3H2![]() 2NH3�ɵã����������������ʵ���֮��Ϊ1:3��Ӧ������N2��H2��ת��������ȵģ����Ƿ�ﵽƽ��״̬�أ���A����B��÷�Ӧ��һ������������仯�ķ�Ӧ����ѹʱֻҪ��ƽ�⣬��������ͻᷢ���仯����Ϊ�������������䣬�����ܶȾͻ�ϱ仯�����ܶȲ���ʱ��˵�����淴Ӧ������ȣ�ƽ�ⲻ�ٷ����ƶ�����B��ȷ��C������Ƿ�Ӧ������������ֻҪ��ƽ�⣬���������٣����������࣬���ֵ
2NH3�ɵã����������������ʵ���֮��Ϊ1:3��Ӧ������N2��H2��ת��������ȵģ����Ƿ�ﵽƽ��״̬�أ���A����B��÷�Ӧ��һ������������仯�ķ�Ӧ����ѹʱֻҪ��ƽ�⣬��������ͻᷢ���仯����Ϊ�������������䣬�����ܶȾͻ�ϱ仯�����ܶȲ���ʱ��˵�����淴Ӧ������ȣ�ƽ�ⲻ�ٷ����ƶ�����B��ȷ��C������Ƿ�Ӧ������������ֻҪ��ƽ�⣬���������٣����������࣬���ֵ![]() �ͻ�仯������ֵ����ʱ˵���Ѿ�����ƽ��״̬����C��ȷ��D������Ƿ�Ӧ������������ֻҪ��ƽ�⣬���������٣����������࣬���ֵ
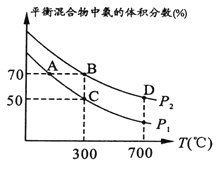
�ͻ�仯������ֵ����ʱ˵���Ѿ�����ƽ��״̬����C��ȷ��D������Ƿ�Ӧ������������ֻҪ��ƽ�⣬���������٣����������࣬���ֵ![]() �ͻ�仯������ֵ����ʱ˵���Ѿ�����ƽ��״̬��������ֵ=2ʱ��ȴ��һ�����ٱ仯�����Բ�һ����ƽ��״̬����D���ںϳɰ���Ӧ��һ�������������С�ķ�Ӧ������ѹǿ������ƽ�������ƶ�����ͼ��֪���¶���ͬʱ��ѹǿΪP1ʱƽ�������а����������С��ѹǿΪP2ʱ������P1<P2���ϳɰ�����ӦΪ���ȷ�Ӧ���¶�Խ��ѧƽ�ⳣ��ԽС��D���¶ȸ���B���¶ȣ����Ի�ѧƽ�ⳣ����B��>D����
�ͻ�仯������ֵ����ʱ˵���Ѿ�����ƽ��״̬��������ֵ=2ʱ��ȴ��һ�����ٱ仯�����Բ�һ����ƽ��״̬����D���ںϳɰ���Ӧ��һ�������������С�ķ�Ӧ������ѹǿ������ƽ�������ƶ�����ͼ��֪���¶���ͬʱ��ѹǿΪP1ʱƽ�������а����������С��ѹǿΪP2ʱ������P1<P2���ϳɰ�����ӦΪ���ȷ�Ӧ���¶�Խ��ѧƽ�ⳣ��ԽС��D���¶ȸ���B���¶ȣ����Ի�ѧƽ�ⳣ����B��>D����
����c��H2��ת����Ϊ��(H2)��������ʽ�ã�
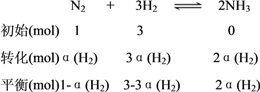
��Ϊƽ�������а����������Ϊ50%��ͬ��ͬѹ�������������=���ʵ�������������![]() ��100%=50%�������(H2)��66.7%����ͼ�ɵã�A��B����ƽ�������а������������ͬ��B���¶Ⱥ�ѹǿ������A���¶Ⱥ�ѹǿ������A��B�������������÷�Ӧ�ӿ�ʼ��ƽ��ʱ���ɰ���ƽ������:v(A)<v(B)��
��100%=50%�������(H2)��66.7%����ͼ�ɵã�A��B����ƽ�������а������������ͬ��B���¶Ⱥ�ѹǿ������A���¶Ⱥ�ѹǿ������A��B�������������÷�Ӧ�ӿ�ʼ��ƽ��ʱ���ɰ���ƽ������:v(A)<v(B)��
��3����NH3��NaClOһ�������·���������ԭ��Ӧ�ɵõ���(N2H4)��NaClO��������������ԭΪNaCl������ԭ���غ㣬����ˮ���ɣ��ʻ�ѧ����ʽΪ��2NH3+NaClO![]() N2H4+NaCl+H2O����K1=
N2H4+NaCl+H2O����K1=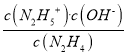 =1��10-a������c(N2H5+)=c(N2H4)ʱ��c(OH-)=1��10-a��c(H+)=
=1��10-a������c(N2H5+)=c(N2H4)ʱ��c(OH-)=1��10-a��c(H+)=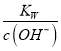 =10-(14-a)��pH=-lgc(H+)=14-a����c(N2H5+)>c(N2H4)ʱ��c(OH-)<1��10-a��c(H+)=
=10-(14-a)��pH=-lgc(H+)=14-a����c(N2H5+)>c(N2H4)ʱ��c(OH-)<1��10-a��c(H+)=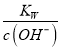 >10-(14-a)��pH=-lgc(H+)<14-a��K2=
>10-(14-a)��pH=-lgc(H+)<14-a��K2=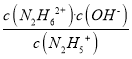 =1��10-b������c(N2H5+)=c(N2H62+)ʱ��c(OH-)=1��10-b��c(H+)=
=1��10-b������c(N2H5+)=c(N2H62+)ʱ��c(OH-)=1��10-b��c(H+)= =10-(14-b)��pH=-lgc(H+)=14-b����c(N2H5+)>c(N2H62+)ʱ��c(OH-)>1��10-b��c(H+)=
=10-(14-b)��pH=-lgc(H+)=14-b����c(N2H5+)>c(N2H62+)ʱ��c(OH-)>1��10-b��c(H+)=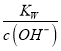 <10-(14-b)��pH=-lgc(H+)>14-b������25��ʱ����N2H4ˮ��Һ�м���H2SO4����ʹc(N2H5+)>c(N2H4)��ͬʱc(N2H5+)>c(N2H62+)��Ӧ������ҺpH��ΧΪ��(14-b��14-a)��
<10-(14-b)��pH=-lgc(H+)>14-b������25��ʱ����N2H4ˮ��Һ�м���H2SO4����ʹc(N2H5+)>c(N2H4)��ͬʱc(N2H5+)>c(N2H62+)��Ӧ������ҺpH��ΧΪ��(14-b��14-a)��

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д�