��Ŀ����
��10�֣�ijһ��Ӧ��ϵ�е������У�HCl��SnCl2��H2SnCl6��As��H3AsO3��H2O,
��֪��As�Dz���֮һ��
��1��д������ƽ�÷�Ӧ�Ļ�ѧ����ʽ ______________
��2���ڸ÷�Ӧ�У��õ��ӵ�������______________ ����������Ԫ����______________��
��3���ڷ�Ӧ�У�ÿת��1 mol���ӣ����ģ������ɣ�HCl_______mol��
��4������������ȷ����_____________����д��ţ���
a������ͬ�����£���VIIA��Ԫ�ص������ӵĻ�ԭ�Դ��ϵ�������ǿ
b��Sn��Pbλ��ͬһ���壬��+4�۵Ļ���������ȶ�
c������ͬ�����£���ԭ��˳��S2-��I-��Fe2+��Br-��Cl-
d. �����з�Ӧ�У������ԣ�SnCl2 ��As��ԭ�ԣ�H3AsO3��H2SnCl6
��֪��As�Dz���֮һ��
��1��д������ƽ�÷�Ӧ�Ļ�ѧ����ʽ ______________
��2���ڸ÷�Ӧ�У��õ��ӵ�������______________ ����������Ԫ����______________��
��3���ڷ�Ӧ�У�ÿת��1 mol���ӣ����ģ������ɣ�HCl_______mol��
��4������������ȷ����_____________����д��ţ���
a������ͬ�����£���VIIA��Ԫ�ص������ӵĻ�ԭ�Դ��ϵ�������ǿ
b��Sn��Pbλ��ͬһ���壬��+4�۵Ļ���������ȶ�
c������ͬ�����£���ԭ��˳��S2-��I-��Fe2+��Br-��Cl-
d. �����з�Ӧ�У������ԣ�SnCl2 ��As��ԭ�ԣ�H3AsO3��H2SnCl6
��1��12HCl+3SnCl2+2H3AsO3 2As+6H2O+3H2SnCl6
��2��H3AsO3 +2��Sn
��3��2 ��4�� ac
��2��H3AsO3 +2��Sn
��3��2 ��4�� ac
��

��ϰ��ϵ�д�
 Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�
�����Ŀ
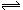 H2CO3 + 2OH-
H2CO3 + 2OH-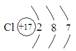
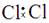
 6Cu + SO2�����ڸ÷�Ӧ������˵���д������
6Cu + SO2�����ڸ÷�Ӧ������˵���д������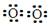 H
H  H3O++ CO32��
H3O++ CO32��