��Ŀ����
����Ŀ��ij��ȤС��ͬѧ������ȩ��������Ӧ��ʵ������������£�
A�����Թ�����ע������NaOH��Һ����Ȼ�������С���NaOH��Һ��ȥ����������ˮϴ���Թܱ��á�
B����ϴ�����Թ�������������Һ��
C�����Թܱڼ�����ȩϡ��Һ��
D�����ȡ���ش��������⣺
��1������A�м�NaOH��Һ��������е�Ŀ����_____________��
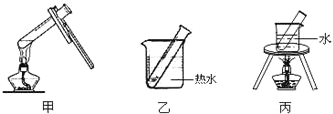
��2������DӦѡ��ļ��ȷ�����_____________��������װ�ñ�ţ�
��3����ȩ����������Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��4������ȤС���ͬѧ������ȩ����������Ӧ�����ʵ������������̽��������ʵ���������±�����

��ʵ��1��ʵ��3��̽������_________________________��
�ڵ�������Һ����Ϊ1 mL����ȩ����Ϊ3�Σ��¶�Ϊ55�棬��Ӧ���ҺpHΪ11ʱ������������ʱ��Ϊ_____________min�����Χ��
������Ϊ̽����ȩ����������Ӧ��������������˲ⶨ�������ֵ�ʱ���⣬����Ҫ�Ƚϲ�ͬ�������γ�������_____________��
���𰸡� ȥ���Թ��ڱڵ����ۣ���֤�Թܽྻ �� CH3CHO��2Ag(NH3)2OH ��CH3COONH4 ��2Ag����3NH3 ��H2O ��ȩ�����Է�Ӧ���ʵ�Ӱ�� 5��6.5 �����̶�
�������������������1��������Ӧ��Ҫ�ྻ���Թܣ���֬��NaOH��Һ�м���ˮ�⡣
��2��������Ӧ��Ҫˮԡ���ȣ�
��3����ȩ����������Ӧ��������李�����������ˮ��
��4����ʵ��1��ʵ��3�Ƚϣ�ʵ��3������ȩ��Ũ�ȡ�
��ʵ��1��ʵ��2�Ƚϣ��¶�Խ�߳��ֳ���������ʱ��Խ�̡�
��̽����ȩ����������Ӧ�������������Ӧ�����⣬����Ҫ�Ƚϲ�ͬ�������γ������Ĺ����̶ȡ�
��������1����֬��NaOH��Һ�м���ˮ�⣬����A�м�NaOH��Һ��������е�Ŀ����ȥ���Թ��ڱڵ����ۣ���֤�Թܽྻ��
��2��������Ӧ��Ҫˮԡ���ȣ���ѡ��װ�ã�
��3����ȩ����������Ӧ��������李�����������ˮ����ѧ����ʽΪCH3CHO��2Ag(NH3)2OH ��CH3COONH4 ��2Ag����3NH3 ��H2O��
��4����ʵ��1��ʵ��3�Ƚϣ�ʵ��3������ȩ������������ʵ��1��ʵ��3��̽��������ȩ�����Է�Ӧ���ʵ�Ӱ�졣
�ڸ���ʵ��1��ʵ��2�Ƚϣ��¶�Խ�߳��ֳ���������ʱ��Խ�̣��¶�Ϊ55�����ʵ��1��ʵ��2֮�䣬���Գ���������ʱ��Ϊʵ��1��ʵ��2֮�䣬Ϊ5��6.5��
��̽����ȩ����������Ӧ�������������Ӧ�����⣬����Ҫ�Ƚϲ�ͬ�������γ������Ĺ����̶ȡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��Ϊ��СCO2�Ի�����Ӱ�죬���������ŷ�����ͬʱ��Ӧ��ǿ��CO2�������õ��о���
(1)�� �Ѻ��нϸ�Ũ��CO2�Ŀ���ͨ�뱥��K2CO3��Һ��
�� ����������Һ��ͨ����ˮ�����õ���Ũ�ȵ�CO2���塣
д�����з�Ӧ�Ļ�ѧ����ʽ________��
(2)�罫CO2��H2 ��1:3������Ȼ�ϡ�
���ʵ������ºϳ�ij����ˮ��������_______(�����)��
A������ B��ϩ�� C��Ȳ�� D������ͬϵ��
�� �ʵ������ºϳ�ȼ�ϼ״���ˮ�������Ϊ2L���ܱ������У�����2 mol CO2��6 mol H2��һ�������·�����Ӧ��CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g) ��H����49.0 kJ/mol��
CH3OH(g)��H2O(g) ��H����49.0 kJ/mol��
���CO2(g)��CH3OH(g)��Ũ����ʱ��仯��ͼ��ʾ��

�ӷ�Ӧ��ʼ��ƽ�⣬v(H2)��______��������ת���ʣ�_______����ʹƽ����ϵ��n(CH3OH)����Ĵ�ʩ��______��
(3)�罫CO2��H2 ��1:4������Ȼ�ϣ����ʵ��������¿��Ƶ�CH4��
��֪��
CH4 (g) + 2O2(g) ![]() CO2(g)+ 2H2O(l) ��H1���D 890.3 kJ/mol
CO2(g)+ 2H2O(l) ��H1���D 890.3 kJ/mol
H2(g) + 1/2O2(g) ![]() H2O(l) ��H2����285.8 kJ/mol
H2O(l) ��H2����285.8 kJ/mol
��CO2(g)��H2(g)��Ӧ����CH4(g)��Һ̬ˮ���Ȼ�ѧ����ʽ��________��
(4)ijͬѧ���������ⶨ���нϸ�Ũ��CO2�Ŀ�����CO2�ĺ����������һЩ������20�����������±���
�ܽ��(S)/g | �ܶȻ�(Ksp) | ||
Ca(OH)2 | Ba(OH)2 | CaCO3 | BaCO3 |
0.16 | 3.89 | 2.9��10-9 | 2.6��10-9 |
(˵����KspԽС����ʾ��������ˮ��Һ��Խ�׳���)
����CO2����ʵ��Լ���_________[����Ca(OH)2������Ba(OH)2��]��Һ��ʵ��ʱ����Ҫ�ⶨ��ҵ���������(����ɱ�״��)�⣬����Ҫ�ⶨ__________��




