��Ŀ����
���ڵؿ��еĺ����ϸߣ��輰�仯����Ŀ��������Ѿã����ִ��������й㷺Ӧ�ã��ش��������⣺
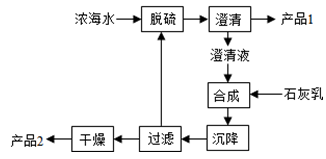
��1���մɡ�ˮ��Ͳ����dz��õĴ�ͳ�����ǽ������ϣ�����������ͨ��������Ҫԭ����______��
��2���ߴ������ִ���Ϣ���뵼��������Ȳ�ҵ����Ҫ�Ļ������ϣ���ҵ���ᴿ���ж���·�ߣ�����һ�ֹ�������ʾ��ͼ����Ҫ��Ӧ��ͼ��
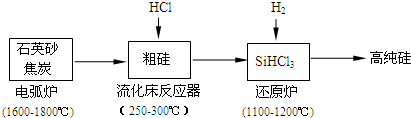
�ٹ�ҵ����ʯӢɰ�ͽ�̿�ڵ绡¯�и��¼��ȵ�1600��-1800������ɴֹ��⣬Ҳ��������̼���裬���ڵ绡¯�ڿ��ܷ����ķ�Ӧ�Ļ�ѧ����ʽΪ______��
������������Ӧ�IJ����У�SiHCl3��Լռ85%������SiCl4��SiH2Cl2��SiH3Cl�ȣ��ֹ�����SiHCl3�Ļ�ѧ��Ӧ����ʽ______��
��3���й����ʵķе��������±����ᴿSiHCl3����Ҫ���ղ��������dz�����������______��SiHCl3����ˮ�⣬����ȫˮ��IJ���Ϊ______��
��4����ԭ¯�з����Ļ�ѧ��ӦΪ��______��
��5���ȼҵ��Ϊ�������������ṩ����ԭ�ϣ���Щԭ����______��
��1���մɡ�ˮ��Ͳ����dz��õĴ�ͳ�����ǽ������ϣ�����������ͨ��������Ҫԭ����______��
��2���ߴ������ִ���Ϣ���뵼��������Ȳ�ҵ����Ҫ�Ļ������ϣ���ҵ���ᴿ���ж���·�ߣ�����һ�ֹ�������ʾ��ͼ����Ҫ��Ӧ��ͼ��

�ٹ�ҵ����ʯӢɰ�ͽ�̿�ڵ绡¯�и��¼��ȵ�1600��-1800������ɴֹ��⣬Ҳ��������̼���裬���ڵ绡¯�ڿ��ܷ����ķ�Ӧ�Ļ�ѧ����ʽΪ______��
������������Ӧ�IJ����У�SiHCl3��Լռ85%������SiCl4��SiH2Cl2��SiH3Cl�ȣ��ֹ�����SiHCl3�Ļ�ѧ��Ӧ����ʽ______��
��3���й����ʵķе��������±����ᴿSiHCl3����Ҫ���ղ��������dz�����������______��SiHCl3����ˮ�⣬����ȫˮ��IJ���Ϊ______��
| ���� | Si | SiCl4 | SiHCl3 | SiH2Cl2 | SiH3Cl | HCl | SiH4 |
| �е�/�� | 2355 | 57.6 | 31.8 | 8.2 | -30.4 | -84.9 | -111.9 |
��5���ȼҵ��Ϊ�������������ṩ����ԭ�ϣ���Щԭ����______��
��1����ҵ��������ͨ��������Ҫԭ���Ǵ��ʯӢ��ʯ��ʯ���ʴ�Ϊ�����ʯӢ��ʯ��ʯ��
��2����ʯӢɰ����Ҫ�ɷ��Ƕ������裬�Ʊ��ֹ跢���û���Ӧ��SiO2+2C
Si+2CO����ͬʱ���ڷ�Ӧ�У�Ҳ��������̼���裬��ӦΪ��SiO2+2C
Si+2CO����SiO2+3C
SiC+2CO����
�ʴ�Ϊ��SiO2+2C
Si+2CO����SiO2+3C
SiC+2CO����
�ڴֹ������HCl���巴ӦSi+3HCl
SiHCl3+H2���ʴ�Ϊ��Si+3HCl
SiHCl3+H2��
��3�����÷е�IJ�ͬ�ᴿSiHCl3��������SiHCl3���е�33.0�棩�к�������SiCl4���е�57.6�棩��HCl���е�-84.7�棩�����ڷе���ϴ���ͨ����������ȥ���ʣ�SiHCl3ˮ�ⷴӦ����ʽΪ��SiHCl3+3H2O�TH2SiO3+H2��+3HCl�������ɹ��ᡢ�������Ȼ��⣬
�ʴ�Ϊ���ʴ�Ϊ����������H4SiO4����H2SiO3����H2��HCl��
��4����ԭ¯��SiHCl3������������Ӧ�Ƶô��裬SiHCl3+H2
Si+3HCl��
�ʴ�Ϊ��SiHCl3+H2
Si+3HCl��
��5���ȼҵ��Ҫ��ӦΪ��ⱥ��ʳ��ˮ��2NaCl+2H2O
2NaOH+H2��+Cl2����Ϊ�û�ѧ�����ṩH2��HCl���ʴ�Ϊ��H2��HCl��
��2����ʯӢɰ����Ҫ�ɷ��Ƕ������裬�Ʊ��ֹ跢���û���Ӧ��SiO2+2C
| ||
| ||
| ||
�ʴ�Ϊ��SiO2+2C
| ||
| ||
�ڴֹ������HCl���巴ӦSi+3HCl
| ||
| ||
��3�����÷е�IJ�ͬ�ᴿSiHCl3��������SiHCl3���е�33.0�棩�к�������SiCl4���е�57.6�棩��HCl���е�-84.7�棩�����ڷе���ϴ���ͨ����������ȥ���ʣ�SiHCl3ˮ�ⷴӦ����ʽΪ��SiHCl3+3H2O�TH2SiO3+H2��+3HCl�������ɹ��ᡢ�������Ȼ��⣬
�ʴ�Ϊ���ʴ�Ϊ����������H4SiO4����H2SiO3����H2��HCl��
��4����ԭ¯��SiHCl3������������Ӧ�Ƶô��裬SiHCl3+H2
| ||
�ʴ�Ϊ��SiHCl3+H2
| ||
��5���ȼҵ��Ҫ��ӦΪ��ⱥ��ʳ��ˮ��2NaCl+2H2O
| ||

��ϰ��ϵ�д�
�����Ŀ
 NaBr + NaBrO3+6NaHCO3������1mol Br2ʱת�Ƶĵ���Ϊ mol��
NaBr + NaBrO3+6NaHCO3������1mol Br2ʱת�Ƶĵ���Ϊ mol��