��Ŀ����
�����й�ʵ��IJ������̣���ȷ���� �� ��
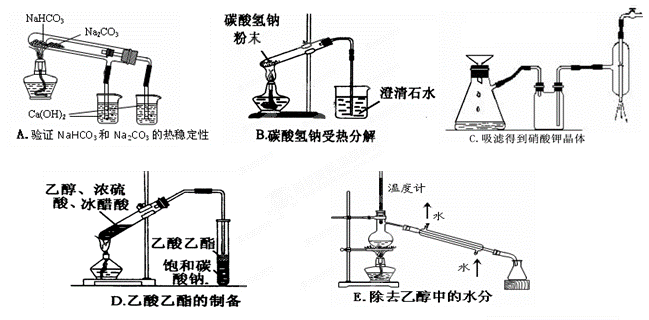
| A������ˮ�ķ�Ӧ�������Ӵ��Լ�ƿ��ȡ�������ƣ���С�������̶�����С��һ����С�ķ���װ��ˮ���ձ��� |
| B������100mL����������10%��H2O2��Һ��ȡһ֧���Ϊ100mL����Ͳ����ȡ��������Ϊ30%��˫��ˮ��Һ33.3mL��Ȼ���ڼ�ˮ��100mL�̶��� |
| C������ij�Ȼ�����Һ�к���Fe2+���ӣ�ȡ����Һ�����������м���KSCN��Һ�������ɫ���������еμ���ˮ����Һ��Ϊ��ɫ�� |
| D������ijδ֪��Һ�Ƿ���K�����ýྻ�IJ�˿պȡ��������Һ���ڻ��������գ�����ɫ�ܲ����۲�������ɫ |
D
��

��ϰ��ϵ�д�
�����Ŀ
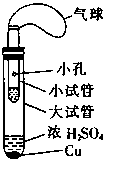

 (2 ) ����ʵ��û�д������_______________________
(2 ) ����ʵ��û�д������_______________________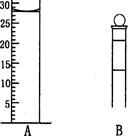

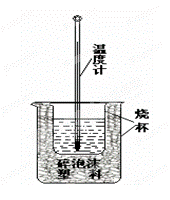

 ��(NH4)2SO4��Һ
��(NH4)2SO4��Һ ��ɫ
��ɫ ���������ձ�
���������ձ�