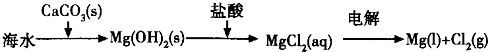
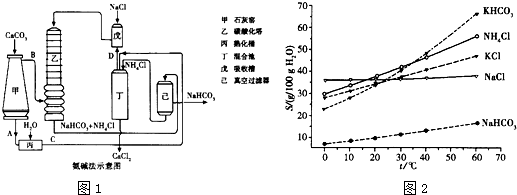
��Ŀ����
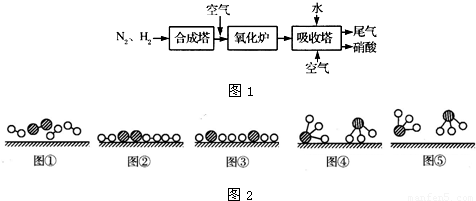
��ͼ1��ʾ�ǹ�ҵ������������̣�

�ϳ�������������ý������¯������Pt-Rh�Ͻ�������ش��������⣺
��1��1909�껯ѧ�ҹ�����ʵ�����״κϳ��˰���2007�껯ѧ�Ҹ����?���ض��ڹ����о���֤ʵ�������뵪���ڹ����������ϳɰ��ķ�Ӧ���̣�ʾ����ͼ2��ʾ�� ��
�� ��
�� �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���
��2���ϳɰ���Ӧ�Ļ�ѧ����ʽΪN2��g��+3H2��g��?2NH3��g����д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK=
��3����֪��4NH3��g��+3O2��g��=2N2��g��+6H2O��g����H=-1 266.8kJ/mol
N2��g��+O2��g��=2NO��g����H=+1 80.5kJ/mol�������������Ȼ�ѧ����ʽΪ
��4����������ͨ�������Ŀ����

�ϳ�������������ý������¯������Pt-Rh�Ͻ�������ش��������⣺
��1��1909�껯ѧ�ҹ�����ʵ�����״κϳ��˰���2007�껯ѧ�Ҹ����?���ض��ڹ����о���֤ʵ�������뵪���ڹ����������ϳɰ��ķ�Ӧ���̣�ʾ����ͼ2��ʾ��
 ��
�� ��
�� �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���ͼ�ڱ�ʾN2��H2�������ڴ������棬ͼ�۱�ʾ�ڴ������棬N2��H2�л�ѧ������
ͼ�ڱ�ʾN2��H2�������ڴ������棬ͼ�۱�ʾ�ڴ������棬N2��H2�л�ѧ������
����2���ϳɰ���Ӧ�Ļ�ѧ����ʽΪN2��g��+3H2��g��?2NH3��g����д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽK=
K=
��
| c2(NH3) |
| c(N2)��c3(H2) |
K=
��
����һ���¶Ⱥ�ѹǿ�£���H2 ��N2 ��3��1�����֮�ȣ���Ϻ����ϳ�������Ӧ�ﵽƽ��ʱ��ƽ��������NH3 ���������Ϊ15%����ʱH2 ��ת����Ϊ| c2(NH3) |
| c(N2)��c3(H2) |
26%
26%
����3����֪��4NH3��g��+3O2��g��=2N2��g��+6H2O��g����H=-1 266.8kJ/mol
N2��g��+O2��g��=2NO��g����H=+1 80.5kJ/mol�������������Ȼ�ѧ����ʽΪ
4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905.8kJ/mol
4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905.8kJ/mol
����4����������ͨ�������Ŀ����
ʹNOѭ�����ã�ȫ��ת��������
ʹNOѭ�����ã�ȫ��ת��������
����������1����ͼ���DZ�ʾN2��H2�������ڴ����ı��棻ͼ�����ʾ�ڴ������棬N2��H2�еĻ�ѧ�����ѣ�
��2�����ٻ�ѧƽ�ⳣ���ǣ����ض����������£����¶ȡ�ѹ�����ܼ����ʡ�����ǿ���ȣ������滯ѧ��Ӧ�ﵽƽ��״̬ʱ�������뷴Ӧ���Ũ�ȱȻ�Ӧ���뷴Ӧ�����Ũ�ȱȣ��÷��š�K����ʾ��
�ڸ��ݷ�Ӧ����ʽ N2��g��+3H2��g��?2NH3��g������μӷ�Ӧ�����������ʵ���xmol��ԭ��������� H2 ��N2 ��3��1�����֮�ȣ���ϣ����ʵ���֮��Ҳ��3��1��������3nmol������nmol����ʽ���㼴�ɣ�
��3�������ø�˹���ɣ��ѵڶ����Ȼ�ѧ����ʽ��������2����Ȼ����������ʽ��ӾͿ��Եõ������������Ȼ�ѧ����ʽ��
��4����ͨ��������ṩ������������Ա�ʹNO�ܹ�ѭ�����ã�ȫ��ת�������ᣮ
��2�����ٻ�ѧƽ�ⳣ���ǣ����ض����������£����¶ȡ�ѹ�����ܼ����ʡ�����ǿ���ȣ������滯ѧ��Ӧ�ﵽƽ��״̬ʱ�������뷴Ӧ���Ũ�ȱȻ�Ӧ���뷴Ӧ�����Ũ�ȱȣ��÷��š�K����ʾ��
�ڸ��ݷ�Ӧ����ʽ N2��g��+3H2��g��?2NH3��g������μӷ�Ӧ�����������ʵ���xmol��ԭ��������� H2 ��N2 ��3��1�����֮�ȣ���ϣ����ʵ���֮��Ҳ��3��1��������3nmol������nmol����ʽ���㼴�ɣ�
��3�������ø�˹���ɣ��ѵڶ����Ȼ�ѧ����ʽ��������2����Ȼ����������ʽ��ӾͿ��Եõ������������Ȼ�ѧ����ʽ��
��4����ͨ��������ṩ������������Ա�ʹNO�ܹ�ѭ�����ã�ȫ��ת�������ᣮ
����⣺��1������������ͼ����֪����ͼ�ڱ�ʾN2��H2�������ڴ������棬��ͼ�۱�ʾ�ڴ������棬N2��H2�л�ѧ�����ѣ��ʴ�Ϊ��ͼ�ڱ�ʾN2��H2�������ڴ������棬ͼ�۱�ʾ�ڴ������棬N2��H2�л�ѧ�����ѣ�
��2������ѧƽ�ⳣ���ǣ����ض����������£����¶ȡ�ѹ�����ܼ����ʡ�����ǿ���ȣ������滯ѧ��Ӧ�ﵽƽ��״̬ʱ�������뷴Ӧ���Ũ�ȱȻ�Ӧ���뷴Ӧ�����Ũ�ȱȣ��������ϸ������д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ K=
���з�Ӧ����ʽ��N2��g��+3H2��g��?2NH3��g������μӷ�Ӧ�����������ʵ���xmol��ԭ��������� H2 ��N2 ��3��1�����֮�ȣ���ϣ����ʵ���֮��Ҳ��3��1��������3nmol������nmol����μӷ�Ӧ�ĵ��������ʵ���Ϊx/3mol�����ɵİ���Ϊ2x/3mol����ʽ��
=15%�����x��0.78nmol��H2 ��ת����Ϊ��0.78nmol��3nmol��100%=26%��
�ʴ�Ϊ��K=
�� 26%��
��3������֪��4NH3��g��+3O2��g��=2N2��g��+6H2O��g����H=-1 266.8kJ/mol ��
N2��g��+O2��g��=2NO��g����H=+1 80.5kJ/mol ��
��+�ڡ�2�ã�4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905.8kJ/mol
�ʴ�Ϊ��4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905.8kJ/mol��
��4��������ͨ��������ṩ������������Ա�ʹNOѭ�����ã�ȫ��ת�������ᣮ
�ʴ�Ϊ��ʹNOѭ�����ã�ȫ��ת�������ᣮ
��2������ѧƽ�ⳣ���ǣ����ض����������£����¶ȡ�ѹ�����ܼ����ʡ�����ǿ���ȣ������滯ѧ��Ӧ�ﵽƽ��״̬ʱ�������뷴Ӧ���Ũ�ȱȻ�Ӧ���뷴Ӧ�����Ũ�ȱȣ��������ϸ������д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ K=
| c2(NH3) |
| c(N2)��c3(H2) |
| 2X/3 |
| n-x/3+3n-x+2x/3 |
�ʴ�Ϊ��K=
| c2(NH3) |
| c(N2)��c3(H2) |
��3������֪��4NH3��g��+3O2��g��=2N2��g��+6H2O��g����H=-1 266.8kJ/mol ��
N2��g��+O2��g��=2NO��g����H=+1 80.5kJ/mol ��
��+�ڡ�2�ã�4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905.8kJ/mol
�ʴ�Ϊ��4NH3��g��+5O2��g��=4NO��g��+6H2O��g����H=-905.8kJ/mol��
��4��������ͨ��������ṩ������������Ա�ʹNOѭ�����ã�ȫ��ת�������ᣮ
�ʴ�Ϊ��ʹNOѭ�����ã�ȫ��ת�������ᣮ
����������������Ĺ�ҵ�Ʊ�Ϊ�������ۺϿ���Ի�ѧ��Ӧ�л�ѧ���仯���Ȼ�ѧ����ʽ����ѧƽ�ⳣ����ת���ʼ���ȿ����Լ���ͼ�εĹ۲�����������������

��ϰ��ϵ�д�
�����Ŀ
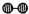 ��
��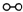 ��
�� �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���______��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���______��
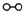

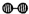 ��
�� ��
�� �ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���______��
�ֱ��ʾN2��H2��NH3��ͼ�ݱ�ʾ���ɵ�NH3�뿪�������棬ͼ�ں�ͼ�۵ĺ���ֱ���______��