��Ŀ����
ij��ɫ��Һ�ֻ��������8�������еļ��֣�Mg2����H����Ag����Na����Cl����HCO3����OH����NO3������֪����Һ�ܸ���������Ӧ���ҷų�������ֻ���������Իش�
(1)����Һ������Ӧֻ��AlO2�����ɣ���ԭ��Һһ�����еĴ�����������________(�ѧʽ)��������Ӧ�����ӷ���ʽ��________________________�������ܺ��еĽ϶��������______________________��
(2)����Һ������Ӧ����Al3�����ɣ���ԭ��Һ��һ�����ܴ������ڵ�������__________________��
���𰸡�(1)NaOH��2Al��2OH����2H2O===2AlO2����3H2����NaCl��NaNO3
(2)HCO3����OH����Ag����NO3��
����������Al��Ӧ����H2�����ֿ��ܣ�һ��������������H������һ��û��HCO3����OH����NO3������ô������Cl����Cl����Ag���ֲ��ܹ��棬��һ��û��Ag������Ϊ������������һ��������OH����һ��û��Mg2����H����Ag����HCO3�������뺬��Na����

��ϰ��ϵ�д�
�����Ŀ
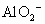 ���ɣ���ԭ��Һ��һ�����д�����________���ӣ��������д�����________���ӣ�
���ɣ���ԭ��Һ��һ�����д�����________���ӣ��������д�����________���ӣ� ���ɣ���ԭ��Һ��һ�������д�����________���ӣ�
���ɣ���ԭ��Һ��һ�������д�����________���ӣ�