��Ŀ����
��8�֣�ȼ�շ��Dzⶨ�л������ﻯѧʽ��һ����Ҫ��������һ���¶���ȡ0.1 molij��̬��A��O2����ȫȼ�գ�����CO2��ˮ�������ų�����Q kJ��������������ͨ��Ũ����ͼ�ʯ�ң�Ũ��������7.2 g����ʯ������17.6 g������A��������KMnO4��Һ��Br2��CCl4��Һ������ʹ������ɫ��
kJ��������������ͨ��Ũ����ͼ�ʯ�ң�Ũ��������7.2 g����ʯ������17.6 g������A��������KMnO4��Һ��Br2��CCl4��Һ������ʹ������ɫ��
��1��д����A�ķ���ʽ ��
��2�� ��֪��A���ӽṹ�߶ȶԳ���������������д����A�Ľṹ��ʽ ��
��3��д����AʹBr2��CCl4��Һ��ɫ�Ļ�ѧ����ʽ ��
��4����A��һ�������¿���ͨ���Ӿ۷�Ӧ�ϳ�һ�����ϣ���÷�Ӧ�Ļ�ѧ����ʽ�� ��
 kJ��������������ͨ��Ũ����ͼ�ʯ�ң�Ũ��������7.2 g����ʯ������17.6 g������A��������KMnO4��Һ��Br2��CCl4��Һ������ʹ������ɫ��
kJ��������������ͨ��Ũ����ͼ�ʯ�ң�Ũ��������7.2 g����ʯ������17.6 g������A��������KMnO4��Һ��Br2��CCl4��Һ������ʹ������ɫ����1��д����A�ķ���ʽ ��
��2�� ��֪��A���ӽṹ�߶ȶԳ���������������д����A�Ľṹ��ʽ ��
��3��д����AʹBr2��CCl4��Һ��ɫ�Ļ�ѧ����ʽ ��
��4����A��һ�������¿���ͨ���Ӿ۷�Ӧ�ϳ�һ�����ϣ���÷�Ӧ�Ļ�ѧ����ʽ�� ��
��8�֣���1��C4H8��2�֣�����2��CH3CH=CHCH3��2�֣���
 ��3��CH3CH=CHCH3+Br2 CH3CHBrCHBrCH3��2�֣���
��3��CH3CH=CHCH3+Br2 CH3CHBrCHBrCH3��2�֣���
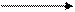


��4��nCH3CH=CHCH3 [CH��CH]n ��2�֣�
 ��3��CH3CH=CHCH3+Br2 CH3CHBrCHBrCH3��2�֣���
��3��CH3CH=CHCH3+Br2 CH3CHBrCHBrCH3��2�֣���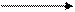


|
|
|
|
��

��ϰ��ϵ�д�
�����Ŀ
 A. nCH2= CH2
A. nCH2= CH2
