��Ŀ����
X��Y��Z��Q��MΪ�����Ķ�����Ԫ�أ���ԭ���������������й���Ϣ���±�:
|
X |
��ֲ����������ȱ�ٵ�Ԫ�أ��ǵ����ʵ���Ҫ�ɷ� |
|
Y |
�ؿ��к����ӵ�һλ |
|
Z |
����������ԭ�Ӱ뾶��� |
|
Q |
�����д���ʹ����Ͻ���Ʒ����ҵ�Ͽ��õ����������ķ����Ʊ� |
|
M |
��ˮ�д���������Ԫ��֮һ������������ϼ��븺�۵Ĵ�����Ϊ6 |
��1��X����̬�⻯��Ĵ���������������˵���������ʳ��������µļ������������⣬��д������̬�⻯��ĵ���ʽ____________��
��2����֪37Rb��53I��λ�ڵ������ڣ��ֱ���Z��Mͬһ���塣�����й�˵����ȷ����____________������ţ���
A��ԭ�Ӱ뾶�� Rb>I
B��RbM�к��й��ۼ�
C����̬�⻯�����ȶ��ԣ�M>I
D��Rb��Q��M������������Ӧ��ˮ�����������������Ӧ
��3��������QX�����Ժã�������ϵ��С�������õ����ȳ�����ϡ������ڽ�����ʴ������ǿ���������������������Ͻ�������������ϡ��йػ�����QX���Ʊ�����ѧ�������£������������ݾ����ۺ�Ϊ25�桢101.3 kPa�����µ���ֵ��
����Q��X�ĵ�����800 ~ 1000���Ƶã�ÿ����1 mol QX������a kJ��������
����Q���������̿��X�ĵ�����1600 ~ 1750������QX��ÿ����1 mol QX������18 g̼������b kJ��������
�����������Ϣд����������Q�����������̿��Ӧ����Q���ʺ�CO���Ȼ�ѧ����ʽ________________��
��4��X��Y��ɵ�һ����ɫ������������Ϊ����ɫ������״����40 �̸���ɫ������15 ������ͨ��һ��Ũ�ȵ�NaOH��Һ�У�ǡ�ñ���ȫ���գ�ͬʱ���������Ρ���д���÷�Ӧ�����ӷ���ʽ ��
����8�֣�
��1��
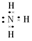
��2��A C D���д����÷֣����2����1�֣�ȫ�Ե�2�֣�
��3��3C��s��+ Al 2O3��s��= 2Al��s��+3CO��g�� ��H= -(2a-2b) kJ/mol �� ��H= 2b -2a kJ/mol
��4��8NO + 3O2 + 8OH- = 2NO- 3+6NO- 2 + 4H2O
��������
��������� ��������Ϣ��֪X��N��Y��O��Z��Na��Q��Al��M��Cl��
��1��NH3�ĵ���ʽ��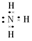
��2��A��ͬ���ڴ�������ԭ�Ӱ뾶�ݼ�������Rb>I����ȷ��B��RbM��ֻ�������Ӽ�������C����̬�⻯�����ȶ�����ǽ�����ǿ���йأ�M>I ����ȷ��D��Rb��Q��M������������Ӧ��ˮ����ֱ�Ϊǿ������������ǿ�ᣬ���Կ�������������Ӧ����ȷ��
��3����������ɵâ�ʽN2��g��+2Al��s��=2 AlN��s�� ��H=+2akJ/mol�� ��ʽ3C��s��+ Al 2O3��s��+ N2��g��= 2 AlN��s��+3CO��g����H=+2bkJ/mol,�ɢ�ʽ-��ʽ�ɵ�Ŀ�귽��ʽ��3C��s��+ Al 2O3��s��= 2Al��s��+3CO��g�� ��H= -(2a-2b) kJ/mol��
��4��X��Y��ɵ�һ����ɫ����ΪNO������������8:3�ı�����Ӧ��8NO + 3O2 + 8OH- = 2NO- 3+6NO- 2 + 4H2O
���㣺������Ԫ���ƶ�Ϊ���忼���˹����Ӧ�á�����ʽ��Ԫ�������ɡ�����ʽ��д�����֪ʶ����

 ������������ϵ�д�
������������ϵ�д�| X | ��ֲ����������ȱ�ٵ�Ԫ�أ��ǵ����ʵ���Ҫ�ɷ� |
| Y | �����������Ǵ�����3�� |
| Z | �������У���ԭ�Ӱ뾶��� |
| Q | �����д���ʹ����Ͻ���Ʒ����ҵ�Ͽ��õ����������ķ����Ʊ� |
| M | ��ˮ�д���������Ԫ��֮һ������������ϼ��븺�۵Ĵ�����Ϊ6 |
| A��ԭ�Ӱ뾶��X��Y��M |
| B����X��M��������Ԫ�ز������γ����ӻ����� |
| C����̬�⻯�����ȶ��ԣ�M��X��Y |
| D��Z��Q��M������������Ӧ��ˮ�����������������Ӧ |
X��Y��Z��Q��MΪ�����Ķ�����Ԫ�أ���ԭ���������������й���Ϣ���±�:
|
X |
��ֲ����������ȱ�ٵ�Ԫ�أ��ǵ����ʵ���Ҫ�ɷ� |
|
Y |
�ؿ��к����ӵ�һλ |
|
Z |
����������ԭ�Ӱ뾶��� |
|
Q |
�����д���ʹ����Ͻ���Ʒ����ҵ�Ͽ��õ����������ķ����Ʊ� |
|
M |
��ˮ�д���������Ԫ��֮һ������������ϼ��븺�۵Ĵ�����Ϊ6 |
��1��X����̬�⻯��Ĵ���������������˵���������ʳ��������µļ������������⣬��д������̬�⻯��ĵ���ʽ____________��
��2����֪37Rb��53I��λ�ڵ������ڣ��ֱ���Z��Mͬһ���塣�����й�˵����ȷ����____________������ţ���
A��ԭ�Ӱ뾶��Rb��I
B��RbM�к��й��ۼ�
C����̬�⻯�����ȶ��ԣ�M��I
D��Rb��Q��M������������Ӧ��ˮ�����������������Ӧ
��3��X��Y��ɵ�һ����ɫ������������Ϊ����ɫ������״����40 �̸���ɫ������15 ������ͨ��һ��Ũ�ȵ�NaOH��Һ�У�ǡ�ñ���ȫ���գ�ͬʱ���������Ρ���д���÷�Ӧ�����ӷ���ʽ ��



