��Ŀ����
��07��ȫ�����������ºʹ���ѹǿ�£���ͼʾ��װ�ý���ʵ�飬���ag�� CaC2 90%����Ʒ��ˮ��ȫ��Ӧ�������������Ϊ b L����������ͬ�����£��ⶨij��ʯ������ CaC2 ��������������ش��������⣺

��1�� CaC2 ��ˮ��Ӧ�Ļ�ѧ����ʽ�� �� ��
��2������Ӧ�ս���ʱ���۲쵽��ʵ��������ͼ��ʾ����ʱ��������ȡ�������ܣ������Ǣ� ��
��3����ʵ���в����������ʱӦע��������� �� ��
��4�������ʯ��������Ϊ c g������������Ϊd L�����ʯ������ CaC2 ��������������ʽ
W�� CaC2 �� = �� ��(���������ɵ�����������Բ���)��
�𰸣�
��1��CaC2+2H2O=Ca(OH)2+C2H2��
��2����Ϊװ����������¶�û�лָ������£�����ѹǿ�������ڴ���ѹǿ��
��3����װ����������¶Ȼָ������º�����Ͳʹ������Һ���ƽ��
��4��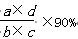
������CaC2��ˮ�ķ�Ӧ�Ƿ��ȷ�Ӧ��ʹװ����������¶ȸ�������¶ȣ��ָ�������֮ǰ�����崦������״̬��װ��������ѹǿ���ڴ���ѹǿ����������ɲ������������ʵ��С������һ������Ҳ������ɲ������������ʵ��С��������ͼ��ʾ����Ͳ��Һ�����ˮ����Һ�棬������������⣬����İ취�ǻָ������º��ٵ�����Ͳʹ������Һ���ƽ��

��07��ȫ��������ϸ�������б������������й��ɣ��ж������ڵ�15λ�����ķ���ʽ�ǣ� ��
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ���� |
C2H2 | C2H4 | C2H6 | C3H4 | C3H6 | C3H8 | C4H6 | C4H8 | C4H10 | ���� |
A��C6H12���� B��C6H14���� �� C��C7H12���� ����D��C7H14
