��Ŀ����
��2014��ӱ�ʡ������ѧ����3�����������ۻ�ѧ�Ծ���
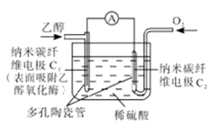
�ȼҵ��������Ļ�ѧ��ҵ֮һ������Ĥ��ⷨΪĿǰ�ձ�ʹ�õ�������������������������ͼ��ʾ��

��1���������п���ѭ���������� ��
��2����ⷨ�Ƽ����Ҫԭ���DZ���ʳ��ˮ�����ڴ���ˮ�к���Ca2+��Mg2+��SO42���������ʣ������ڽ������ǰ��Ҫ�������ξ��ƣ�д��һ�ξ����з��������ӷ���ʽ ����ʳ��ˮ���������ξ��ƾ�ֱ�ӽ�������Ĥ���ۻ����ʲô��� ��
��3����ͼ�ǹ�ҵ�ϵ�ⱥ��ʳ��ˮ�����ӽ���Ĥ����ʾ��ͼ(�����ý��������Ƴɣ�������̼�����Ƴ�)����B�������������� ��E�缫�������� ������ܷ�Ӧ�����ӷ���ʽΪ ��

��4���������۳����ĵ���ˮ�У����������������ܽ��ȣ���Ҫ����8����9��������������Һ���䳹�׳�ȥ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5����֪�ڵ����У�ÿСʱͨ��1�����ֱ������Բ���1.492g���ռij������300�����۴�������8Сʱ���Ƶ�32%���ռ���Һ���ܶ�Ϊ1.342��/m3��113m3�����۵ĵ���ǿ��1.45 ��104A���õ��۵ĵ��Ч��Ϊ ��
�ȼҵ��������Ļ�ѧ��ҵ֮һ������Ĥ��ⷨΪĿǰ�ձ�ʹ�õ�������������������������ͼ��ʾ��

��1���������п���ѭ���������� ��
��2����ⷨ�Ƽ����Ҫԭ���DZ���ʳ��ˮ�����ڴ���ˮ�к���Ca2+��Mg2+��SO42���������ʣ������ڽ������ǰ��Ҫ�������ξ��ƣ�д��һ�ξ����з��������ӷ���ʽ ����ʳ��ˮ���������ξ��ƾ�ֱ�ӽ�������Ĥ���ۻ����ʲô��� ��
��3����ͼ�ǹ�ҵ�ϵ�ⱥ��ʳ��ˮ�����ӽ���Ĥ����ʾ��ͼ(�����ý��������Ƴɣ�������̼�����Ƴ�)����B�������������� ��E�缫�������� ������ܷ�Ӧ�����ӷ���ʽΪ ��

��4���������۳����ĵ���ˮ�У����������������ܽ��ȣ���Ҫ����8����9��������������Һ���䳹�׳�ȥ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5����֪�ڵ����У�ÿСʱͨ��1�����ֱ������Բ���1.492g���ռij������300�����۴�������8Сʱ���Ƶ�32%���ռ���Һ���ܶ�Ϊ1.342��/m3��113m3�����۵ĵ���ǿ��1.45 ��104A���õ��۵ĵ��Ч��Ϊ ��
��1���Ȼ��� ��������
��2��Ca2++ CO32�� = CaCO3�� Mg2+ + 2OH�� = Mg(OH)2�� ���Լ����������ˮ�л���������Mg2+��Ca2+�����������»����ɳ����������ӽ���Ĥ��
��3��H2������ 2Cl����2H2O Cl2����H2����2 OH��
Cl2����H2����2 OH��
��4��Na2SO3 + Cl2 + H2O = Na2SO4 + 2HCl
��5��93.46%
��2��Ca2++ CO32�� = CaCO3�� Mg2+ + 2OH�� = Mg(OH)2�� ���Լ����������ˮ�л���������Mg2+��Ca2+�����������»����ɳ����������ӽ���Ĥ��
��3��H2������ 2Cl����2H2O
 Cl2����H2����2 OH��
Cl2����H2����2 OH����4��Na2SO3 + Cl2 + H2O = Na2SO4 + 2HCl
��5��93.46%
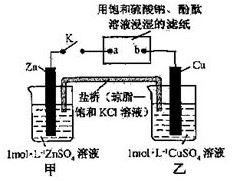
�ȼҵ�ĵ��ԭ����2Cl����2H2O
 Cl2����H2����2OH����(1)��ͼ�Ͽ�δ����NaCl�Ͳ���NaOH����ѭ��ʹ�á���2������ˮ�к���Ca2+��Mg2+��SO42���������ʣ�һ�ξ���ʱ���봿����ռ����CO32-ʹCa2+������OH��ʹMg2+���������Լ����������ˮ�л���������Mg2+��Ca2+����ʳ��ˮ���������ξ��ƾ�ֱ�ӽ�������Ĥ���ۣ����������»����ɳ����������ӽ���Ĥ��
Cl2����H2����2OH����(1)��ͼ�Ͽ�δ����NaCl�Ͳ���NaOH����ѭ��ʹ�á���2������ˮ�к���Ca2+��Mg2+��SO42���������ʣ�һ�ξ���ʱ���봿����ռ����CO32-ʹCa2+������OH��ʹMg2+���������Լ����������ˮ�л���������Mg2+��Ca2+����ʳ��ˮ���������ξ��ƾ�ֱ�ӽ�������Ĥ���ۣ����������»����ɳ����������ӽ���Ĥ����3��ͼ��Na+�����ͨ�����ӽ���Ĥ�����Ҳ࣬˵��F�缫ΪH+�ŵ磬��������B��������������H2��
E�缫������������ܷ�Ӧ�����ӷ���ʽ�����ԭ����
��4���ܽ���������������Һ��Ӧ��Cl2���Cl-���������Ʊ�������ƣ�ע��ˮ�����˷�Ӧ��
��5��
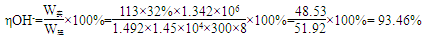

��ϰ��ϵ�д�
 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�
�����Ŀ