��Ŀ����
̼���京̼�����������ǵ�����������Ӧ�ù㷺��
��1����Ȼ���������ճ������е������Դ��
��֪��CH4��g����2O2��g����CO2��g����2 H2O��l���� ��H1����890��3kJ��mol��1
��2 CO��g����O2��g����2CO2 (g)�� ��H2����566 kJ��mol��1
��Ӧ2 CH4��g����3O2��g����2CO��g����4H2O��l���ġ�H��_____________��
��2������ȼ�ϵ�صĻ�ѧ����ʽΪCH4��2O2��CO2��2H2O��ij����ȼ�ϵ���Լ���Ϊȼ�ϣ��Կ���Ϊ�������������ڵ�K2CO3�����в���O2����HCO3����Ϊ����ʣ��Ծ��д����ú͵������ܵ�ϡ������Ϊ�缫��
��ȼ�ϵ�صĸ����缫��ӦʽΪ��CH4��8e����4CO32����5CO2��2H2O�����������缫��ӦʽΪ____________��Ϊʹ����ʵ���ɱ����ȶ���ʹ��ȼ�ϵ�س�ʱ���ȶ����У���ͨ��Ŀ����б������________________���ʡ�
��3���Ը�ȼ�ϵ��Ϊ��Դ���Բ����缫���1000g 4��55����NaOH��Һ��һ��ʱ�����Һ�����ʵ�����������Ϊ5��00���������������������ڱ�״���µ����Ϊ________L��
��4������ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2 L�ĺ����ܱ������У����з�ӦCO��g����H2O��g�� CO2��g����H2��g�����õ������������ݣ�
CO2��g����H2��g�����õ������������ݣ�
��ʵ��A����v��H2����ʾ�ķ�Ӧ����Ϊ___________________��
��ͨ�������֪��CO��ת����ʵ��A_______________ʵ��B������ڡ��������ڡ���С�ڡ������÷�Ӧ������ӦΪ______________�ȷ�Ӧ��������š�����
����ʵ��CҪ�ﵽ��ʵ��B��ͬ��ƽ��״̬����a��bӦ����Ĺ�ϵ��_________________���ú�a��b����ѧʽ��ʾ����
��1����Ȼ���������ճ������е������Դ��
��֪��CH4��g����2O2��g����CO2��g����2 H2O��l���� ��H1����890��3kJ��mol��1
��2 CO��g����O2��g����2CO2 (g)�� ��H2����566 kJ��mol��1
��Ӧ2 CH4��g����3O2��g����2CO��g����4H2O��l���ġ�H��_____________��
��2������ȼ�ϵ�صĻ�ѧ����ʽΪCH4��2O2��CO2��2H2O��ij����ȼ�ϵ���Լ���Ϊȼ�ϣ��Կ���Ϊ�������������ڵ�K2CO3�����в���O2����HCO3����Ϊ����ʣ��Ծ��д����ú͵������ܵ�ϡ������Ϊ�缫��
��ȼ�ϵ�صĸ����缫��ӦʽΪ��CH4��8e����4CO32����5CO2��2H2O�����������缫��ӦʽΪ____________��Ϊʹ����ʵ���ɱ����ȶ���ʹ��ȼ�ϵ�س�ʱ���ȶ����У���ͨ��Ŀ����б������________________���ʡ�
��3���Ը�ȼ�ϵ��Ϊ��Դ���Բ����缫���1000g 4��55����NaOH��Һ��һ��ʱ�����Һ�����ʵ�����������Ϊ5��00���������������������ڱ�״���µ����Ϊ________L��
��4������ͬ����CO��g����H2O��g���ֱ�ͨ�뵽���Ϊ2 L�ĺ����ܱ������У����з�ӦCO��g����H2O��g��
 CO2��g����H2��g�����õ������������ݣ�
CO2��g����H2��g�����õ������������ݣ�
��ʵ��A����v��H2����ʾ�ķ�Ӧ����Ϊ___________________��
��ͨ�������֪��CO��ת����ʵ��A_______________ʵ��B������ڡ��������ڡ���С�ڡ������÷�Ӧ������ӦΪ______________�ȷ�Ӧ��������š�����
����ʵ��CҪ�ﵽ��ʵ��B��ͬ��ƽ��״̬����a��bӦ����Ĺ�ϵ��_________________���ú�a��b����ѧʽ��ʾ����
����15�֣�
��1����1214.6 kJ��mol-1��2�֣�
��2�� O2 + 4e- + 2CO2 = 2CO32- CO2��4�֣�ÿ��2�֣�
��3�� 56 L��3�֣�
��4���� 0.16 mol ?? L -1 ?? min -1��2�֣�
�� ���� �ţ�2�֣�ÿ��1�֣�
�� b="2a " ��2�֣�
��1����1214.6 kJ��mol-1��2�֣�
��2�� O2 + 4e- + 2CO2 = 2CO32- CO2��4�֣�ÿ��2�֣�
��3�� 56 L��3�֣�
��4���� 0.16 mol ?? L -1 ?? min -1��2�֣�
�� ���� �ţ�2�֣�ÿ��1�֣�
�� b="2a " ��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
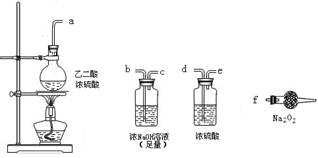
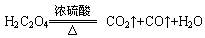 ����ʵ��ѡ������װ�ýӿ�����˳��Ϊ________��
����ʵ��ѡ������װ�ýӿ�����˳��Ϊ________��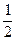 O2(g)=CO2(g)����H="-283.0" kJ��mol -1��д��CO2��C(s)��Ӧ���Ȼ�ѧ����ʽ___________________����COΪȼ������ȼ�ϵ�أ���ص�����ͨ��O2��CO2������ͨ��CO�������������̼���Σ��ŵ�ʱ������ӦʽΪ______________________����ʹ�øõ�ص�ⱥ��ʳ��ˮ��ȡ1molNaClO������������Ҫ���������Ϊ����״���£�________L��
O2(g)=CO2(g)����H="-283.0" kJ��mol -1��д��CO2��C(s)��Ӧ���Ȼ�ѧ����ʽ___________________����COΪȼ������ȼ�ϵ�أ���ص�����ͨ��O2��CO2������ͨ��CO�������������̼���Σ��ŵ�ʱ������ӦʽΪ______________________����ʹ�øõ�ص�ⱥ��ʳ��ˮ��ȡ1molNaClO������������Ҫ���������Ϊ����״���£�________L�� aOH ��6��Si��H2
aOH ��6��Si��H2