��Ŀ����
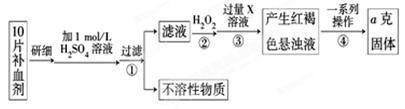
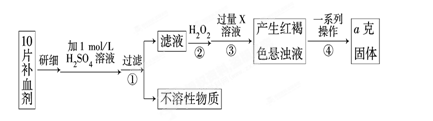
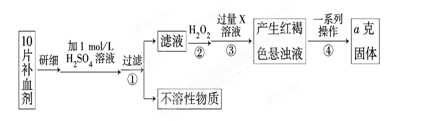
��10�֣���2010��8��7�գ�����ʡ�����ط����ش���ʯ�����صľ�Ԯ����ԶԶ����������������ͼΪ�ط�������һ�ֹ����ֺŲ�����Ѫ��ҩ����������������Ҷ��Ƭ������Ҫ�ɷ����̷����������������壨FeSO4��7H2O��
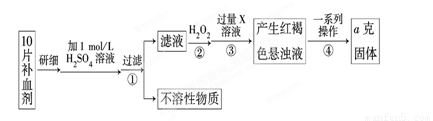
��ijУ����С��Ϊ�ⶨ�ò�Ѫ������Ԫ�صĺ��������ʵ�鲽�����£�
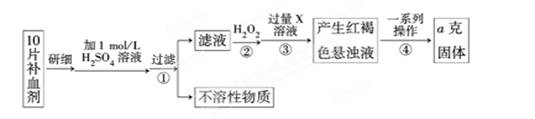
��ش��������⣺
��1��������м������H2O2��Ŀ���� ��
��2��������з�Ӧ�����ӷ���ʽΪ ��
��3���������һϵ�д����IJ��������ǣ����ˡ� �����ա� ��������
��4����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����Ϊ g���ú�a�Ĵ���ʽ��ʾ����
�����ѹ�������������ˮ��÷dz��Ļ��ǣ��������á���˶���Ⱦ������ˮ�Ĵ������˷dz���Ҫ���£����д����ķ����кࣺܶ
��1�������������̷�������һ����������Ⱦ������ˮ�����Ϻõľ�ˮЧ���������ǣ������ӷ���ʽ��ʾ�� ���������������������������������������������� ��
��2����ʹ�ù����з����̷��������Ļ��ʹ�ö����Է�ˮ�е������ﴦ��Ч���ϲ��ԭ���� ��
��1������Һ��Fe2����ȫת��ΪFe3����1�֣�
��2��Fe3����3OH��===Fe(OH)3����Fe3����3NH3��H2O===Fe(OH)3����3NH��1�֣�
��3��ϴ�ӣ�1�֣� ��ȴ��1�֣�
��4��0.07a��2�֣�
��1��Cl2��H2O H����Cl�D��HClO Cl2��2Fe2��=2Fe3����2Cl��
H����Cl�D��HClO Cl2��2Fe2��=2Fe3����2Cl��
Fe3����3H2O Fe(OH)3�����壩��3H��������������д��һ����1�֣���3�֣�
Fe(OH)3�����壩��3H��������������д��һ����1�֣���3�֣�
��2�������Է�Һ�У���������Fe3����ˮ�⣬���õ�Fe(OH)3���壬�ʲ��ܹ��������1�֣�
����������1������Ҫ���ɺ��ɫ������������������˫��ˮ�������ǽ���Һ��Fe2����ȫת��ΪFe3����
��2���÷�Ӧ�������������������ģ����Է���ʽΪFe3����3OH��===Fe(OH)3����Fe3����3NH3��H2O===Fe(OH)3����3NH��
��3�����˺����ϴ�ӡ����պ������ȴ��Ȼ����ܳ�����
��4��ag�����������ѣ����е���Ԫ�ص�������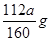 ��0.07ag��
��0.07ag��
��1�������������������ԣ��ܰ����������������������ӡ�������ˮ�������������������������ˮ�е�������Ӷ��ﵽ��ˮ��Ŀ�ġ��йصķ���ʽΪCl2��H2O H����Cl�D��HClO Cl2��2Fe2��=2Fe3����2Cl�� Fe3����3H2O
H����Cl�D��HClO Cl2��2Fe2��=2Fe3����2Cl�� Fe3����3H2O Fe(OH)3�����壩��3H����
Fe(OH)3�����壩��3H����
��2������������ˮ�������ԣ����������Է�Һ�У���������Fe3����ˮ�⣬���õ�Fe(OH)3���壬�ʲ��ܹ��������

 ���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�
���ɶ���ܲ��¿�ֱͨ�п�ϵ�д�