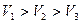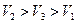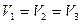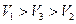��Ŀ����
��������������Һ��ÿ�����������Һ����A��B��C˳�����У�
�� �� �� ��
A.�£�ã��� �Σ����ã��� �ˣã� �����ã���
B.���� ���� ���� ����
C.�Σ����ã��� ����Σ��� �����ã��� �£�ã���
��������в��������۲����������������⣺
��CaCl����Һ�м���A��Һ������ɫ�������������������B��Һ������ʧ����������ɫ��ζ�����壻�ټ���������C��Һ�������ɰ�ɫ������
(1)�������������У�ѡ���� ����Һ��
(2)�йط�Ӧ�����ӷ���ʽ�� ��
�� �� �� ��
A.�£�ã��� �Σ����ã��� �ˣã� �����ã���
B.���� ���� ���� ����
C.�Σ����ã��� ����Σ��� �����ã��� �£�ã���
��������в��������۲����������������⣺
��CaCl����Һ�м���A��Һ������ɫ�������������������B��Һ������ʧ����������ɫ��ζ�����壻�ټ���������C��Һ�������ɰ�ɫ������
(1)�������������У�ѡ���� ����Һ��
(2)�йط�Ӧ�����ӷ���ʽ�� ��
(1)��
(2)�ã��������ã�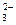
 �ã�ã�����
�ã�ã�����
�ã�ã����������� �ã��������ã�������������
��������������������
����������� �����
�����
(2)�ã��������ã�
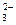
 �ã�ã�����
�ã�ã������ã�ã�����������
 ��������������������
�������������������������������
 �����
����ã����ã�ã�����Һ�м���A������ɫ������������Һ���ҡ����������ã�ã��������������������B��Һ���ҡ��������ϣ��ټ�������C��Һ�������ɰ�ɫ��������ֻ���ҷ��ϣ������C֮ǰ����Һ�к��Уã����������ģ�������Ӧ��������ã���������������ϣ�����Һ�в�����£�������ϳɳ����ģã�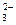 ��
��
 ��
��
��ϰ��ϵ�д�
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
�����Ŀ
 ��Ϊ17g����H2R��Ħ�������� ����������NH3��H2R�����ʵ�����Ϊ ��1.7g������ mol H2O���еĵ�������ȡ�
��Ϊ17g����H2R��Ħ�������� ����������NH3��H2R�����ʵ�����Ϊ ��1.7g������ mol H2O���еĵ�������ȡ�

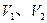 ��
��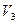 ����
����