��Ŀ����
(1)���鵰�ǣ���Ҫ�ɷ���CaCO3�������������л�����һ����ʹ����ʯ��ˮ����ǵ���ɫ���塣������������Ӧ�����ӷ���ʽΪ����
��
(2)���dz�����ˮƿ�п���ˮ��������Ҫ�ɷ���CaCO3��Mg(OH)2��Ҫ��ȥ ˮ�������õķ�������ƿ�м���ϡ���Ὣ���ܽ⣬�䷴Ӧ�����ӷ���ʽΪ��
ˮ�������õķ�������ƿ�м���ϡ���Ὣ���ܽ⣬�䷴Ӧ�����ӷ���ʽΪ��
��
(3)MgSO4��Һ�еμ�������ˮ���䷴Ӧ�����ӷ���ʽΪ
(4)�뽫�������ӷ���ʽ��дΪ��ѧ����ʽ��
�� 2NH4+ + Ba2+ + 2OH- + SO42- = BaSO4��+2NH3��H2O
�� BaCO3 + 2H+ = Ba2++CO2��+H2O
��
(2)���dz�����ˮƿ�п���ˮ��������Ҫ�ɷ���CaCO3��Mg(OH)2��Ҫ��ȥ
 ˮ�������õķ�������ƿ�м���ϡ���Ὣ���ܽ⣬�䷴Ӧ�����ӷ���ʽΪ��
ˮ�������õķ�������ƿ�м���ϡ���Ὣ���ܽ⣬�䷴Ӧ�����ӷ���ʽΪ�� ��
(3)MgSO4��Һ�еμ�������ˮ���䷴Ӧ�����ӷ���ʽΪ
(4)�뽫�������ӷ���ʽ��дΪ��ѧ����ʽ��
�� 2NH4+ + Ba2+ + 2OH- + SO42- = BaSO4��+2NH3��H2O
�� BaCO3 + 2H+ = Ba2++CO2��+H2O
�� CaCO3 + 2H+ = Ca2+ + CO2��+H2O
�� CO2 + Ca2+ + 2OH�� = CaCO3��+ H2O
�Ƣ� CaCO3 + 2CH3COOH = Ca2+ + 2CH3COO�� +CO2��+H2O
�� Mg(OH)2 + 2CH3COOH = Mg2+ + 2CH3COO�� +2 H2O
�� Mg2++2 NH3��H2O =Mg(OH)2��+2NH4+
�� �� (NH4)2SO4 + Ba(OH)2 = BaSO4��+2 NH3��H2O
�� BaCO3 + 2HCl = BaCl2 + H2O + CO2��������������Ҳ�ɣ�����2�֣�
�� CO2 + Ca2+ + 2OH�� = CaCO3��+ H2O
�Ƣ� CaCO3 + 2CH3COOH = Ca2+ + 2CH3COO�� +CO2��+H2O
�� Mg(OH)2 + 2CH3COOH = Mg2+ + 2CH3COO�� +2 H2O
�� Mg2++2 NH3��H2O =Mg(OH)2��+2NH4+
�� �� (NH4)2SO4 + Ba(OH)2 = BaSO4��+2 NH3��H2O
�� BaCO3 + 2HCl = BaCl2 + H2O + CO2��������������Ҳ�ɣ�����2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
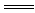 Cu(OH)2����2NaCl
Cu(OH)2����2NaCl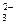 ʮ2H��
ʮ2H��