��Ŀ����
����Ŀ��ij����С��������CuO ��NH3��Ӧ���о�NH3��ij�����ʲ��ⶨ����ɣ����������ʵ��װ�����г�װ��δ����������ʵ�顣��ش��������⡣
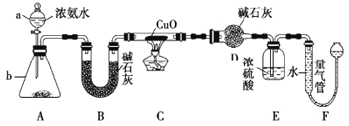
��1�� ����a ������Ϊ___________������b �п�ѡ����Լ�Ϊ____________��
��2��ʵ�����У�����װ��A��������ȡ����ɫ������____________������ĸ����
A��C12 B��O2 C��CO2 D��NO2
��3��ʵ���й۲쵽װ��C �к�ɫCuO��ĩ��Ϊ��ɫ���壬������������ɫ��ζ�������������������֤��NH3����_____________�ԣ�д����Ӧ�Ļ�ѧ����_____________��
��4��װ��E��Ũ�����������______________��
��5����ȡ�������ǰ��Ӧ��װ��F ���еIJ�����________________��
��6��ʵ����ϣ�����ø����D����mg��װ��F�����������ΪnL��������ɱ�״�����������е������ԭ�Ӹ�����ֵΪ____________���ú�m��n��ĸ�Ĵ���ʽ��ʾ����
���𰸡�
��1����Һ©������ʯ���������ƻ��������ƹ�����ʯ������
��2��BC����3����ԭ��3CuO+2NH3===3Cu+3H2O+N2��
��4������δ��Ӧ�İ�������ֹF��ˮ��������D��
��5�����������ƶ��ұ�©����ʹ��������Һ����ƽ����6��![]()
��������
�����������1��װ��������aΪ��Һ©��������b�����÷�Һ©���е���İ�ˮʹ��ƿ�еĹ����ܽ���ȴٽ�һˮ�ϰ��ֽ����ɰ������������ƹ��塢�����ƹ��塢��ʯ�ҹ��壬�ʴ�Ϊ����Һ©���������������ƻ������ƻ��ʯ�ң�
��2������װ��A������ȡ����ɫ���壻A���Ʊ�����Cl2��Ҫ���ȣ�������Ϊ����ɫ���壬��A�����ϣ�B�������Ʊ�O2�����ù���������ƺ�ˮ�ķ�Ӧ����B���ϣ�C�������Ʊ�CO2 ���壬����ϡ����������ʯ�Ϸ�Ӧ���ɣ���C���ϣ�D��NO2�Ǻ���ɫ���壬��D�����ϣ���ѡBC��
��3��ʵ���й۲쵽װ��C�к�ɫCuO��ĩ��Ϊ��ɫ���壬����������ɫ��ζ�����壬˵������������ͭ��Ӧ����ͭ�͵�����ˮ������������ͭ�������ֻ�ԭ�ԣ����ԭ���غ���ƽд���Ļ�ѧ����ʽΪ��3CuO+2NH3![]() 3Cu+3H2O+N2 ���ʴ�Ϊ����ԭ��3CuO+2NH3
3Cu+3H2O+N2 ���ʴ�Ϊ����ԭ��3CuO+2NH3![]() 3Cu+3H2O+N2 ��
3Cu+3H2O+N2 ��
��4���������̷�����Ũ���������չ����İ�������ֹF��ˮ��������DӰ��ʵ��Ч�����ʴ�Ϊ������δ��Ӧ�İ�������ֹF��ˮ��������D��
��5����ȡ�������ǰ��Ӧ��װ��F���еIJ��������������ƶ��ұ�©����ʹ��������Һ����ƽ������ѹǿƽ���ٶ������ʴ�Ϊ�����������ƶ��ұ�©����ʹ��������Һ����ƽ��
��6������ø����D����mgΪˮ���ʵ���=![]() ��װ��F�����������ΪnL��������ɱ�״����ΪN2�����ʵ���=
��װ��F�����������ΪnL��������ɱ�״����ΪN2�����ʵ���=![]() ������Ԫ���غ�õ���ԭ�Ӻ���ԭ�����ʵ���֮��=
������Ԫ���غ�õ���ԭ�Ӻ���ԭ�����ʵ���֮��=![]() ��2��
��2��![]() ��2=
��2=![]() �������е������ԭ�Ӹ�����Ϊ
�������е������ԭ�Ӹ�����Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�