��Ŀ����
�ҹ�ij���͵��ͭ������ҵ����ұ��������ͭ��������ʴﵽ97����87������ͼ��ʾ��ұ���ӹ������̣�
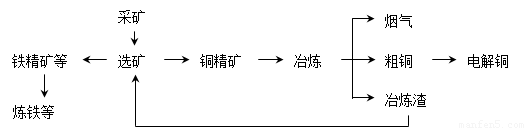
ұ���е���Ҫ��Ӧ��Cu2S + O2 = 2Cu + SO2
��1�������е���Ҫ������________________���������Դ�����ʺͼ��ſ��ǣ����ۺ����÷�ʽ����___________��
��2����ⷨ��ͭʱ��������____________�����ͭ�塱��ͭ�塱������ͭ�к��еĽ����Ե��ʵ���ʽ�ڵ���_______________������������������IJ۵ף������ĵ缫��Ӧʽ��_________________________________________��
��3���ھ���ͭ�Ĺ����У��������Һ��c(Fe2+)��c(Zn2+)���������Ӱ���һ����⡣
�������ʵ��ܶȻ�������KSP����
|
���� |
Fe(OH)2 |
Fe(OH)3 |
Zn(OH)2 |
Cu(OH)2 |
|
KSP |
8.0��10��16 |
4.0��10��38 |
3.0��10��17 |
2.2��10��20 |
���ڵ��Һ��pH�dz�ȥ�������ӵij��÷����������ϱ����ܶȻ������жϣ����е����ʵ���Ũ�ȵ�Fe2+��Zn2+��Fe3+��Cu2+����Һ����pH�������ȳ���������������______________��
һ�ַ������ȼ��������H2O2���ٵ���pH��4���ҡ�����H2O2������Ӧ�����ӷ���ʽΪ___________________________________________________________________________��
��1��SO2 ��1�֣� ���ᣨ1�֣�
��2����ͭ�壨1�֣� ������1�֣� Cu2+ + 2e�� = Cu ��2�֣�
��3��Fe3+ ��2�֣� 2Fe2+ + H2O2 + 2H+ = 2Fe3+ + 2H2O��2�֣�
��������
�����������1���������֪��������Ҫ����ΪSO2���ɻ���������ȡH2SO4�����Ϊ������2����ⷨ��ͭʱ����ͭ������������ͭ����������������ͭ��ʧȥ���ӣ�������������Ӧ��Ҳ�����ڵ��Һ�����ױ�Ϊ�����ࣻ������ӦʽΪCu2+ + 2e�� = Cu ����3���ܶȻ�ԽС������������ܽ��һ��ԽС��Խ�����γɹ�������Һ��������������ͼ��֪��Fe3+���ȳ�����Fe2+���������ɵ����غ㡢����غ㡢ԭ���غ�ԭ����ƽ�ɵã�2Fe2+ + H2O2 + 2H+ = 2Fe3+ + 2H2O��
���㣺���⿼��������仯�������Ҫ���ʼ��Ʊ��ȡ�

 ��У����ϵ�д�
��У����ϵ�д��ҹ�ij���͵��ͭ������ҵ����ұ��������ͭ��������ʴﵽ97����87������ͼ��ʾ��ұ���ӹ������̣�Ks5u
 |
ұ���е���Ҫ��Ӧ��Cu2S + O2 ====== 2Cu + SO2
��1�������е���Ҫ������________________���������Դ�����ʺͼ��ſ��ǣ����ۺ����÷�ʽ����___________��
��2����ⷨ��ͭʱ��������____________�����ͭ�塱��ͭ�塱������ͭ�к��еĽ����Ե��ʵ���ʽ�ڵ���_______________������������������IJ۵ף������ĵ缫��Ӧʽ��_________________________________________��
��3���ھ���ͭ�Ĺ����У��������Һ��c(Fe2+)��c(Zn2+)���������Ӱ���һ����⡣
�������ʵ��ܶȻ�������KSP����
| ���� | Fe(OH)2 | Fe(OH)3 | Zn(OH)2 | Cu(OH)2 |
| KSP | 8.0��10��16 | 4.0��10��38 | 3.0��10��17 | 2.2��10��20 |
���ڵ��Һ��pH�dz�ȥ�������ӵij��÷����������ϱ����ܶȻ������жϣ����е����ʵ���Ũ�ȵ�Fe2+��Zn2+��Fe3+��Cu2+����Һ����pH�������ȳ���������������______________��
һ�ַ������ȼ��������H2O2���ٵ���pH��4���ҡ�����H2O2������Ӧ�����ӷ���ʽΪ___________________________________________________________��
