��Ŀ����
��2013?���ݶ�ģ����ҵ��������Ĺ������£�����ˮ
��Һ
����
����
���������գ�
��1������ˮ������������Mg2+��Ca2+�������������A��B�����ʣ�A��Դ��ʯ��Ҥ�������������B�Ļ�ѧʽΪ
��2��ʵ����ģ������Һ�Ʊ�������װ�����£�

��ͼ1��װ�ú�ͼ2��װ�õ����ӷ���Ϊa��
��ͼ2���Լ�ƿ�ڷ����Ļ�ѧ��Ӧ����ʽΪ
��ʵ����Ҫ��ͨ���NH3����֮����ͨ��CO2���壬����ͨ���NH3�ѹ�����ʵ�������
��3������=5\*MERGEFORMAT �����պ�Ĵ����к���δ�ֽ��̼�����ƣ�ijͬѧ��ȡ�ô�����Ʒm g���ٳ�ּ������������ٱ仯ʱ�Ƶ�ʣ����������Ϊn g������Ʒ��̼���Ƶ���������Ϊ
��100%
��100%��
��4������25���£�0.1mol/LNH3?H2O��Һ��0.1mol/LNH4Cl��Һ����������Һ�������ϲ����Һ��pH=9������˵����ȷ����
A��0.1mol/L NH4Cl��Һ���Ϻ���Һ�е������ӵ��������Ŀ����ͬ
b����Ϻ����Һ�У�c��NH3?H2O����c��Cl-����c��NH4+����c��OH-����c��H+��
c���������֪��NH3?H2O�ĵ���̶ȴ���ͬŨ�ȵ�NH4Cl��ˮ��̶�
d�����ǰ������Һ��pH֮�ʹ���14��
| ���Ⱥ���������A��B |
| �ڹ��� |
| ���Ⱥ�ͨ��NH3CO2 |
| �ܹ��� |
| ������ |
���������գ�
��1������ˮ������������Mg2+��Ca2+�������������A��B�����ʣ�A��Դ��ʯ��Ҥ�������������B�Ļ�ѧʽΪ
Na2CO3
Na2CO3
����2��ʵ����ģ������Һ�Ʊ�������װ�����£�

��ͼ1��װ�ú�ͼ2��װ�õ����ӷ���Ϊa��
d
d
��b��e
e
��f��c����ͼ2���Լ�ƿ�ڷ����Ļ�ѧ��Ӧ����ʽΪ
NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl
����ʵ����Ҫ��ͨ���NH3����֮����ͨ��CO2���壬����ͨ���NH3�ѹ�����ʵ�������
��պ��Ũ����IJ����������ܿ�f�����а������ɣ�˵����������������ʪ��ĺ�ɫʯ����ֽ�����ܿ�f������ֽ������˵����������
��պ��Ũ����IJ����������ܿ�f�����а������ɣ�˵����������������ʪ��ĺ�ɫʯ����ֽ�����ܿ�f������ֽ������˵����������
����3������=5\*MERGEFORMAT �����պ�Ĵ����к���δ�ֽ��̼�����ƣ�ijͬѧ��ȡ�ô�����Ʒm g���ٳ�ּ������������ٱ仯ʱ�Ƶ�ʣ����������Ϊn g������Ʒ��̼���Ƶ���������Ϊ
| 84n-53m |
| 31m |
| 84n-53m |
| 31m |
��4������25���£�0.1mol/LNH3?H2O��Һ��0.1mol/LNH4Cl��Һ����������Һ�������ϲ����Һ��pH=9������˵����ȷ����
cd
cd
������ţ���A��0.1mol/L NH4Cl��Һ���Ϻ���Һ�е������ӵ��������Ŀ����ͬ
b����Ϻ����Һ�У�c��NH3?H2O����c��Cl-����c��NH4+����c��OH-����c��H+��
c���������֪��NH3?H2O�ĵ���̶ȴ���ͬŨ�ȵ�NH4Cl��ˮ��̶�
d�����ǰ������Һ��pH֮�ʹ���14��
��������1����������AԴ��ʯ��Ҥ��˵��������ʯ�һ���ʯ���ҳ�����A��B��������������
��2���ٸ��ݲ������̣�����ʳ��ˮ��ͨ�������̼������Ӧ����̼�����ƣ�
�ڸ��ݲ������̣�����ʳ��ˮ��ͨ�������̼������Ӧ����̼�����ƺ�NH4Cl��
�۸��ݼ��鰱���ķ�����
��3������̼�����������ֽ��̼���������ȶ����������ò�������������ش�
��4��A��0.1mol/L NH4Cl��Һ�е������ӵ������3�֣�Cl-��NH4+��H+�������Һ�е������ӵ������3�֣�Cl-��NH4+��0H-��Cl-��NH4+��H+����NH4+����Ŀ���ȣ�
b��笠����ӵ�ˮ���������ڰ�ˮ�ĵ�����������Ϻ����Һ�У�c��NH3?H2O����c��Cl-����c��NH4+����c��OH-����c��H+��
c��0.1mol/LNH3?H2O��Һ��0.1mol/LNH4Cl��Һ����������Һ�������ϲ����Һ��pH=9��˵��笠����ӵ�ˮ������С�ڰ�ˮ�ĵ���������
d��0.1mol/LNH3?H2O��Һ�Լ��Ժ�0.1mol/LNH4Cl��Һ�����ԣ�笠����ӵ�ˮ������С�ڰ�ˮ�ĵ������������ǰ������Һ��pH֮�ʹ���14��
��2���ٸ��ݲ������̣�����ʳ��ˮ��ͨ�������̼������Ӧ����̼�����ƣ�
�ڸ��ݲ������̣�����ʳ��ˮ��ͨ�������̼������Ӧ����̼�����ƺ�NH4Cl��
�۸��ݼ��鰱���ķ�����
��3������̼�����������ֽ��̼���������ȶ����������ò�������������ش�
��4��A��0.1mol/L NH4Cl��Һ�е������ӵ������3�֣�Cl-��NH4+��H+�������Һ�е������ӵ������3�֣�Cl-��NH4+��0H-��Cl-��NH4+��H+����NH4+����Ŀ���ȣ�
b��笠����ӵ�ˮ���������ڰ�ˮ�ĵ�����������Ϻ����Һ�У�c��NH3?H2O����c��Cl-����c��NH4+����c��OH-����c��H+��
c��0.1mol/LNH3?H2O��Һ��0.1mol/LNH4Cl��Һ����������Һ�������ϲ����Һ��pH=9��˵��笠����ӵ�ˮ������С�ڰ�ˮ�ĵ���������
d��0.1mol/LNH3?H2O��Һ�Լ��Ժ�0.1mol/LNH4Cl��Һ�����ԣ�笠����ӵ�ˮ������С�ڰ�ˮ�ĵ������������ǰ������Һ��pH֮�ʹ���14��
����⣺��1��������AԴ��ʯ��Ҥ��˵��������ʯ�һ���ʯ�ң������е�þ����һ���ü��ȥ��������һ����̼���Ƴ�ȥ���ʴ�Ϊ��Na2CO3��
��2���ٲ������̣��ఱ����ʳ��ˮ��ͨ�������̼������Ӧ����̼�����ƣ�����a��d��b��e��f��c���ʴ�Ϊ��d��e��
������ʳ��ˮ��ͨ�������̼������Ӧ����̼�����ƺ�NH4Cl��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl���ʴ�Ϊ��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��
�ۼ��鰱���ķ�����պ��Ũ����IJ������������а������ɣ���������ʪ��ĺ�ɫʯ������ֽ�������ʴ�Ϊ����պ��Ũ����IJ����������ܿ�f�����а������ɣ�˵����������������ʪ��ĺ�ɫʯ����ֽ�����ܿ�f������ֽ������˵������������
��3���������ǰ���������Ϊm g�����Ⱥ������Ϊn g���������ʧ������Ϊ��m-n������̼�����Ƶ�����Ϊ��84��m-n��/31���ʴ����к��е�̼�����Ƶ���������Ϊ
��100%���ʴ�Ϊ��
��100%��
��4��A��0.1mol/L NH4Cl��Һ�е������ӵ������3�֣�Cl-��NH4+��H+�������Һ�е������ӵ������3�֣�Cl-��NH4+��0H-��Cl-��NH4+��H+����NH4+����Ŀ���ȣ���a����
b��笠����ӵ�ˮ���������ڰ�ˮ�ĵ�����������Ϻ����Һ�У�c��NH3?H2O����c��Cl-����c��NH4+����c��OH-����c��H+������b����
c��0.1mol/LNH3?H2O��Һ��0.1mol/LNH4Cl��Һ����������Һ�������ϲ����Һ��pH=9��˵��笠����ӵ�ˮ������С�ڰ�ˮ�ĵ�����������c��ȷ��
d��0.1mol/LNH3?H2O��Һ�Լ��Ժ�0.1mol/LNH4Cl��Һ�����ԣ�笠����ӵ�ˮ������С�ڰ�ˮ�ĵ������������ǰ������Һ��pH֮�ʹ���14����d��ȷ��
��ѡ��cd��
��2���ٲ������̣��ఱ����ʳ��ˮ��ͨ�������̼������Ӧ����̼�����ƣ�����a��d��b��e��f��c���ʴ�Ϊ��d��e��
������ʳ��ˮ��ͨ�������̼������Ӧ����̼�����ƺ�NH4Cl��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl���ʴ�Ϊ��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��
�ۼ��鰱���ķ�����պ��Ũ����IJ������������а������ɣ���������ʪ��ĺ�ɫʯ������ֽ�������ʴ�Ϊ����պ��Ũ����IJ����������ܿ�f�����а������ɣ�˵����������������ʪ��ĺ�ɫʯ����ֽ�����ܿ�f������ֽ������˵������������
��3���������ǰ���������Ϊm g�����Ⱥ������Ϊn g���������ʧ������Ϊ��m-n������̼�����Ƶ�����Ϊ��84��m-n��/31���ʴ����к��е�̼�����Ƶ���������Ϊ
| 84n-53m |
| 31m |
| 84n-53m |
| 31m |
��4��A��0.1mol/L NH4Cl��Һ�е������ӵ������3�֣�Cl-��NH4+��H+�������Һ�е������ӵ������3�֣�Cl-��NH4+��0H-��Cl-��NH4+��H+����NH4+����Ŀ���ȣ���a����
b��笠����ӵ�ˮ���������ڰ�ˮ�ĵ�����������Ϻ����Һ�У�c��NH3?H2O����c��Cl-����c��NH4+����c��OH-����c��H+������b����
c��0.1mol/LNH3?H2O��Һ��0.1mol/LNH4Cl��Һ����������Һ�������ϲ����Һ��pH=9��˵��笠����ӵ�ˮ������С�ڰ�ˮ�ĵ�����������c��ȷ��
d��0.1mol/LNH3?H2O��Һ�Լ��Ժ�0.1mol/LNH4Cl��Һ�����ԣ�笠����ӵ�ˮ������С�ڰ�ˮ�ĵ������������ǰ������Һ��pH֮�ʹ���14����d��ȷ��
��ѡ��cd��
���������⿼�������ʵ��ᴿ����ѧʵ���������������Ũ�ȵıȽϣ��ѶȲ����ݿα�֪ʶ���ɽ��

��ϰ��ϵ�д�
�����Ŀ

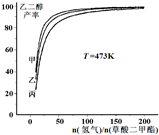 ��������/n�������������]��ѹǿ�ı仯��ϵ�������������߷ֱ��ʾ��ϵѹǿΪ1.5MPa��2.5MPa��3.5MPa�������������Ӧ��ѹǿ��P���ף�=
��������/n�������������]��ѹǿ�ı仯��ϵ�������������߷ֱ��ʾ��ϵѹǿΪ1.5MPa��2.5MPa��3.5MPa�������������Ӧ��ѹǿ��P���ף�=

