��Ŀ����
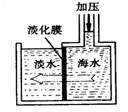
��10�֣��������������գ���Ŀǰ����ˮ���塱������Ҫ����֮һ���乤���������£�
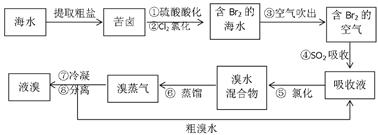
��1���������ڱ���λ��_________���ڣ�_________�塣
��2������ܵ����ӷ���ʽ��________________________________________ ��
��3���������������У�������¶�Ϊ��Ҫ������80��90�档�¶ȹ�����Ͷ������������������ԭ��:___________________________________________ ��
��4���������������������õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��롣����ʵ���ҷ������������ķ���������������___________������ʱҺ��ӷ�������_________����Ͽڡ����¿ڡ����ų���
��5��Ϊʲô��ֱ���ú���ĺ�ˮ��������õ�Һ�壬��Ҫ����������������SO2���ա��Ȼ����� ��

��1���������ڱ���λ��_________���ڣ�_________�塣
��2������ܵ����ӷ���ʽ��________________________________________ ��
��3���������������У�������¶�Ϊ��Ҫ������80��90�档�¶ȹ�����Ͷ������������������ԭ��:___________________________________________ ��
��4���������������������õ�Һ������ˮ�Ļ������������ǵ�����ܶ����ܴ���ص���з��롣����ʵ���ҷ������������ķ���������������___________������ʱҺ��ӷ�������_________����Ͽڡ����¿ڡ����ų���
��5��Ϊʲô��ֱ���ú���ĺ�ˮ��������õ�Һ�壬��Ҫ����������������SO2���ա��Ȼ����� ��
��1������ ��A��2�֣�
��2��Br2+SO2+2H2O=4H++2Br�D+ SO42�D��2�֣�
��3���¶ȹ��ߣ�����ˮ�����ų���������ˮ���ӣ�
�¶ȹ��ͣ��岻����ȫ�����������ʵ͡� ��2�֣�
��4����Һ©�� �¿� ��2�֣�
��5���Ȼ���ĺ�ˮ��Ȼ�����嵥�ʣ���Ũ�ȵͣ����ֱ������ԭ�ϣ���Ʒ�ɱ���
������������SO2���ա��Ȼ����Ĺ���ʵ������һ��Br2��Ũ�����̡���2�֣�
��

��ϰ��ϵ�д�
 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�
�����Ŀ


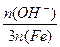 �� 100% ��ʽ��n(OH-)��n(Fe)�ֱ��ʾ����ۺ���������OH����FeԪ�ص����ʵ�������ش��������⣺
�� 100% ��ʽ��n(OH-)��n(Fe)�ֱ��ʾ����ۺ���������OH����FeԪ�ص����ʵ�������ش��������⣺