��Ŀ����
˹̹����ѧB��M��Trost�����������ɫ��ѧ�ĺ��ĸ����ԭ�Ӿ����ԡ����dz���ԭ����������������ѧ��Ӧ���̵�ԭ�Ӿ����ԣ�����㹫ʽΪ��
���������Ϲ��շ��Ʊ���������ˮ������ClO2���䷴Ӧԭ����������ɣ�
�ٵ���Ȼ�����ҺNaCl+3H2O NaClO3+3H2��
NaClO3+3H2��
�������������ϳ��Ȼ���
�����ɶ�������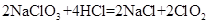 ��+
��+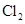 ��+
��+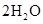
�˷�����ԭ�����������Ϊ
A��37��6 B��53��6% C��62��2% D��94��4%
D
����

��ϰ��ϵ�д�
 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
�����Ŀ
|
˹̹����ѧB��M��Trost�����������ɫ��ѧ�ĺ��ĸ����ԭ�Ӿ����ԣ����dz���ԭ����������������ѧ��Ӧ���̵�ԭ�Ӿ����ԣ�����㹫ʽΪ��
���������Ϲ��շ��Ʊ���������ˮ������ClO2���䷴Ӧԭ����������ɣ� �ٵ���Ȼ�����ҺNaCl��3H2O �������������ϳ��Ȼ��� �����ɶ�������2NaClO3��4HCl��2NaCl��2ClO2����Cl2����2H2O �˷�����ԭ�����������Ϊ | |
| [����] | |
A�� |
37.6 |
B�� |
53.6�� |
C�� |
62.2�� |
D�� |
94.4�� |

 NaClO3+3H2�� �������������ϳ��Ȼ���
NaClO3+3H2�� �������������ϳ��Ȼ���