��Ŀ����
(15��)һ�������£������Ϊ3 L���ܱ������У�һ����̼��������Ӧ���ɼ״�������ΪCu2O/ZnO����
CO(g)��2H2(g) CH3OH(g)
��������������и��⣺
��1����Ӧ�ﵽƽ��ʱ��ƽ�ⳣ������ʽK��
�������¶ȣ�Kֵ �����������С�����䡱����
��2����500�棬�ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)��
��3���������������������£��Դ���E�����ϵ���ѹ����ԭ����1/2�������йظ���ϵ��˵����ȷ����
a. ������Ũ�ȼ��� b. ����Ӧ���ʼӿ죬�淴Ӧ����Ҳ�ӿ�
c. �״������ʵ������� d. ����ƽ��ʱn(H2)/n(CH3OH)����
��4�����о�����Ӧ������������õ�ΪCu2O����Ӧ��ϵ�к�����CO2������ά�ִ���Cu2O�������䣬ԭ���ǣ� ���û�ѧ����ʽ��ʾ����
��1��K��c(CH3OH)/c(CO)��c2(H2) ��С
��2��2nB/3tBmol��(L��min)��1
��3��b c
��4��Cu2O��CO2Cu��CO
����:

��ϰ��ϵ�д�
�����Ŀ
 2SO3(g)����H��0����Ӧ������SO2��O2��SO3���ʵ����仯��ͼ��ʾ��
2SO3(g)����H��0����Ӧ������SO2��O2��SO3���ʵ����仯��ͼ��ʾ��
 2SO3(g)����H��0����Ӧ������SO2��O2��SO3���ʵ����仯��ͼ��ʾ��
2SO3(g)����H��0����Ӧ������SO2��O2��SO3���ʵ����仯��ͼ��ʾ��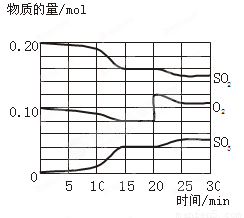
 CH3OH(g)
CH3OH(g)