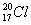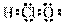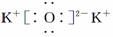��Ŀ����
��������һ�ֳ����Ľ����� �ܺ�Cl2��S��O2��H2O�����ʷ�Ӧ���ش��������⣺
��1��д��Fe��ˮ�����ڸ����·�Ӧ�Ļ�ѧ����ʽ �������������� ��
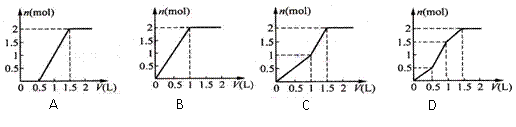
��2����������8����������10����ԭ�ӵķ����� ��
��3��S2-�Ľṹʾ��ͼ�� ��H2O�ĵ���ʽ�� ��
��4����Fe��Ͷ�뵽��������Һ�У�д����Ӧ�����ӷ���ʽ ���ڸ�¯�����У�������ʯ��ԭ�ɵ���������Ҫ��ԭ���� ����Ӧ�Ļ�ѧ����ʽ�� ��
��1��3Fe+4H2O(g)=Fe3O4+4H2(�����Ǹ���)��2�֣� H2O��1�֣�
��2�� 818O ��1�֣� ��3���� ����2�֣�
��4��Fe+2Fe3+=3Fe2+ ��2�֣� CO(1��) Fe2O3+3CO=2Fe+3CO2(�����Ǹ���)(2��)

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ