��Ŀ����
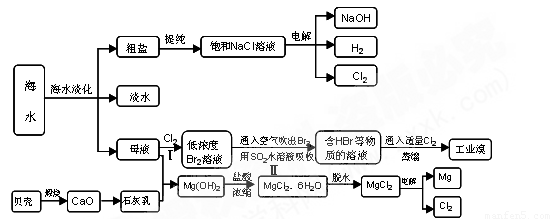
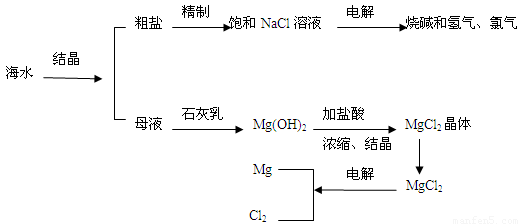
(7��)��ˮ�����౦�����Ȼ��Դ���Ӻ�ˮ�п�����ȡ���ֻ���ԭ�ϡ���ͼ��ij�����Ժ�ˮ��Դ�ۺ����õ�ʾ��ͼ��
(1)�����к���Ca2+��Mg2+��SO42�������ʣ����ƺ�ɵ�NaCl������Һ������ʱͨ��������Һ�����μ��������BaCl2��Һ��������NaOH��Һ������Na2CO3��Һ�����˳�������������Һ������������Һ�����ԡ���д�����в������йػ�ѧ��Ӧ�����ӷ���ʽ��
������Һ�м��������Na2CO3��Һ��__________________��_____________________��
�ڵ���Ȼ�����Һ��______________________________________________��
(2)ĸҺ�к���K+��Na+��Mg2+�������ӣ���ͼ�п��Կ�������ĸҺ����һϵ�еļӹ����Ƶý���þ��
�ٴ����ӷ�Ӧ�ĽǶ�˼������ĸҺ�м���ʯ��������������ǣ��������ӷ�Ӧ����ʽ�ش�___________________________��
�ڼ������������������Ȼ�þ����õ���ˮ�Ȼ�þ��____________________________��
(7��)
(1) ��Ba2+ + CO32��== BaCO3���� Ca2+ + CO32��== CaCO3������2�֣�
��2Cl��+2H2O2OH-+H2��+Cl2����2�֣�
(2) ��Ca(OH)2+Mg2+==Mg(OH)2+Ca2+ (2��)
�� ��HCl�����Χ�¼��� (1��)
����:

 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�