��Ŀ����
��2012?���Ķ�ģ��MMA����һ�ָ߷��ӻ�����л��������ĵ��壬������ȡ����ϩ������ȵ���Ҫԭ�ϣ����������Ʊ��߷��ӣ�MMA����;�������������磺
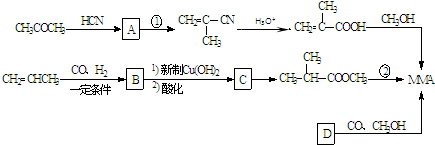
��1����ҵ�Ͻ�A�ͼ״�����������һ��һ����Ӧ����MMA���÷�Ӧ�Ļ�ѧ����ʽΪ
��2����Ӧ������500�沢�д������ڵ������·����ģ����䷴Ӧ����Ϊ��
��3��ij���ʼ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ��������л�������̼ԭ�ӣ�����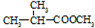 ��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��
��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��
��4������D�ĺ˴Ź��������������壬����CO��CH3OH�����ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ
��5��MMA��1-������Ӧ��ȡ����ϩ������Ļ�ѧ����ʽΪ��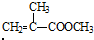 +CH3CH2CH2OH��
+CH3CH2CH2OH��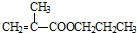 +CH3OH
+CH3OH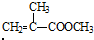 +CH3CH2CH2OH��
+CH3CH2CH2OH��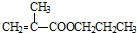 +CH3OH��
+CH3OH��
��6�����������������ṩ������Ϣ��д���ɣ�CH3��2C=CH2�Ʊ�������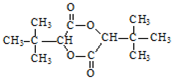 �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ����ͼ��
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ����ͼ��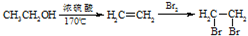

��1����ҵ�Ͻ�A�ͼ״�����������һ��һ����Ӧ����MMA���÷�Ӧ�Ļ�ѧ����ʽΪ
��CH3��2C��OH��CN+CH3OH+H2SO4��CH2�TC��CH3��COOCH3+NH4HSO4
��CH3��2C��OH��CN+CH3OH+H2SO4��CH2�TC��CH3��COOCH3+NH4HSO4
����2����Ӧ������500�沢�д������ڵ������·����ģ����䷴Ӧ����Ϊ��
��ȥ��Ӧ
��ȥ��Ӧ
����3��ij���ʼ��ܷ���������Ӧ�����ܷ���ˮ�ⷴӦ��������л�������̼ԭ�ӣ�����
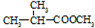 ��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��
��Ϊͬ���칹�壬�������ʽṹ��ʽΪ��HCOOCH��CH3��CH2CH3
HCOOCH��CH3��CH2CH3
����4������D�ĺ˴Ź��������������壬����CO��CH3OH�����ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ
CH��CCH3
CH��CCH3
���÷������ŵ���ԭ��������Ϊ100%
ԭ��������Ϊ100%
����5��MMA��1-������Ӧ��ȡ����ϩ������Ļ�ѧ����ʽΪ��
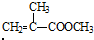 +CH3CH2CH2OH��
+CH3CH2CH2OH��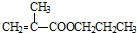 +CH3OH
+CH3OH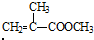 +CH3CH2CH2OH��
+CH3CH2CH2OH��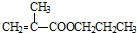 +CH3OH
+CH3OH��6�����������������ṩ������Ϣ��д���ɣ�CH3��2C=CH2�Ʊ�������
 �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ����ͼ��
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ����ͼ��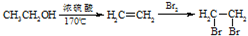
��������ͪ��HCN�����ӳɷ�Ӧ����A��A�Ľṹ��ʽΪ����CH3��2C��OH��CN��A������ȥ��Ӧ����CH2=C��CH3��CN��CH2=C��CH3��CN��ˮ��Ӧ����CH2=C��CH3��COOH��CH2=C��CH3��COOH�ͼ״���Ӧ����MMA��MMA�Ľṹ��ʽΪ��CH2=C��CH3��COOCH3����һ�������£���ϩ��һ����̼��������Ӧ����B��B�Ľṹ��ʽΪ��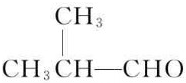 ��B�ܱ�����������ͭ������Ȼ���ữ����C��C�Ľṹ��ʽΪ��
��B�ܱ�����������ͭ������Ȼ���ữ����C��C�Ľṹ��ʽΪ��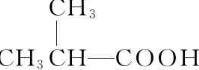 ��C�ͼ״���Ӧ����2-�����������2-���������������ȥ��Ӧ����MMA��D��һ����̼���״������ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ��CH��CCH3��������ʵĽṹ���������������
��C�ͼ״���Ӧ����2-�����������2-���������������ȥ��Ӧ����MMA��D��һ����̼���״������ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ��CH��CCH3��������ʵĽṹ���������������
 ��B�ܱ�����������ͭ������Ȼ���ữ����C��C�Ľṹ��ʽΪ��
��B�ܱ�����������ͭ������Ȼ���ữ����C��C�Ľṹ��ʽΪ��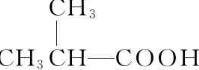 ��C�ͼ״���Ӧ����2-�����������2-���������������ȥ��Ӧ����MMA��D��һ����̼���״������ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ��CH��CCH3��������ʵĽṹ���������������
��C�ͼ״���Ӧ����2-�����������2-���������������ȥ��Ӧ����MMA��D��һ����̼���״������ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ��CH��CCH3��������ʵĽṹ�������������������⣺��ͪ��HCN�����ӳɷ�Ӧ����A��A�Ľṹ��ʽΪ����CH3��2C��OH��CN��A������ȥ��Ӧ����CH2=C��CH3��CN��CH2=C��CH3��CN��ˮ��Ӧ����CH2=C��CH3��COOH��CH2=C��CH3��COOH�ͼ״���Ӧ����MMA��MMA�Ľṹ��ʽΪ��CH2=C��CH3��COOCH3����һ�������£���ϩ��һ����̼��������Ӧ����B��B�Ľṹ��ʽΪ�� ��B�ܱ�����������ͭ������Ȼ���ữ����C��C�Ľṹ��ʽΪ��
��B�ܱ�����������ͭ������Ȼ���ữ����C��C�Ľṹ��ʽΪ��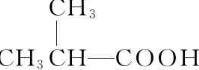 ��C�ͼ״���Ӧ����2-�����������2-���������������ȥ��Ӧ����MMA��D��һ����̼���״������ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ��CH��CCH3��
��C�ͼ״���Ӧ����2-�����������2-���������������ȥ��Ӧ����MMA��D��һ����̼���״������ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ��CH��CCH3��
��1��A�ͼ״�����������һ��һ����Ӧ����MMA�����ڸ÷�Ӧ�������������������������臨���������泥���÷�Ӧ����ʽΪ����CH3��2C��OH��CN+CH3OH+H2SO4��CH2�TC��CH3��COOCH3+NH4HSO4���ʴ�Ϊ����CH3��2C��OH��CN+CH3OH+H2SO4��CH2�TC��CH3��COOCH3+NH4HSO4��
��2��2-�����������500�沢�д������ڵ������·�����ȥ��Ӧ����MMA���ʴ�Ϊ����ȥ��Ӧ��
��3����2-�����������Ϊͬ���칹�壬�Ҹ������к���ȩ��������������̼ԭ�ӣ���������ʽ֪����������Ϊ��������������ʵĽṹ��ʽΪ��HCOOCH��CH3��CH2CH3��
�ʴ�Ϊ��HCOOCH��CH3��CH2CH3��
��4��ͨ�����Ϸ���֪��D�Ľṹ��ʽΪ��CH��CCH3���÷�Ӧ��û���������������ɣ�����ԭ��������Ϊ100%���ʴ�Ϊ��CH��CCH3��ԭ��������Ϊ100%��
��5��MMA��1-��������ȡ����Ӧ���ɼ���ϩ������ͼ״������Ը÷�Ӧ�Ļ�ѧ����ʽΪ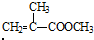 +CH3CH2CH2OH��
+CH3CH2CH2OH��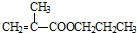 +CH3OH��
+CH3OH��
�ʴ�Ϊ��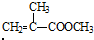 +CH3CH2CH2OH��
+CH3CH2CH2OH��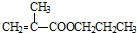 +CH3OH��
+CH3OH��
��6���÷�Ӧ�ϳ�·��Ϊ��2-����ϩ��һ����̼��������Ӧ����2��2-������ȩ��2��2-������ȩ��HCN�����ӳɷ�Ӧ����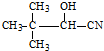 ��
��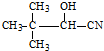 ��Ũ���������·�Ӧ����
��Ũ���������·�Ӧ����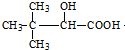 ��
��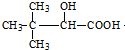 �������Ӽ�������Ӧ����
�������Ӽ�������Ӧ����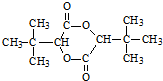 ��
��
�ʴ�Ϊ�� ��
��
 ��B�ܱ�����������ͭ������Ȼ���ữ����C��C�Ľṹ��ʽΪ��
��B�ܱ�����������ͭ������Ȼ���ữ����C��C�Ľṹ��ʽΪ��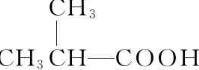 ��C�ͼ״���Ӧ����2-�����������2-���������������ȥ��Ӧ����MMA��D��һ����̼���״������ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ��CH��CCH3��
��C�ͼ״���Ӧ����2-�����������2-���������������ȥ��Ӧ����MMA��D��һ����̼���״������ʵ���֮��1��1��1��Ӧǡ������MMA����D�Ľṹ��ʽΪ��CH��CCH3����1��A�ͼ״�����������һ��һ����Ӧ����MMA�����ڸ÷�Ӧ�������������������������臨���������泥���÷�Ӧ����ʽΪ����CH3��2C��OH��CN+CH3OH+H2SO4��CH2�TC��CH3��COOCH3+NH4HSO4���ʴ�Ϊ����CH3��2C��OH��CN+CH3OH+H2SO4��CH2�TC��CH3��COOCH3+NH4HSO4��
��2��2-�����������500�沢�д������ڵ������·�����ȥ��Ӧ����MMA���ʴ�Ϊ����ȥ��Ӧ��
��3����2-�����������Ϊͬ���칹�壬�Ҹ������к���ȩ��������������̼ԭ�ӣ���������ʽ֪����������Ϊ��������������ʵĽṹ��ʽΪ��HCOOCH��CH3��CH2CH3��
�ʴ�Ϊ��HCOOCH��CH3��CH2CH3��
��4��ͨ�����Ϸ���֪��D�Ľṹ��ʽΪ��CH��CCH3���÷�Ӧ��û���������������ɣ�����ԭ��������Ϊ100%���ʴ�Ϊ��CH��CCH3��ԭ��������Ϊ100%��
��5��MMA��1-��������ȡ����Ӧ���ɼ���ϩ������ͼ״������Ը÷�Ӧ�Ļ�ѧ����ʽΪ
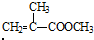 +CH3CH2CH2OH��
+CH3CH2CH2OH��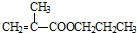 +CH3OH��
+CH3OH���ʴ�Ϊ��
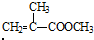 +CH3CH2CH2OH��
+CH3CH2CH2OH��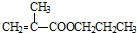 +CH3OH��
+CH3OH����6���÷�Ӧ�ϳ�·��Ϊ��2-����ϩ��һ����̼��������Ӧ����2��2-������ȩ��2��2-������ȩ��HCN�����ӳɷ�Ӧ����
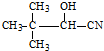 ��
��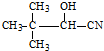 ��Ũ���������·�Ӧ����
��Ũ���������·�Ӧ����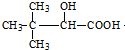 ��
��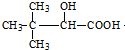 �������Ӽ�������Ӧ����
�������Ӽ�������Ӧ���� ��
���ʴ�Ϊ��
 ��
�����������⿼���л�����ƶϣ���Ŀ�Ѷ��еȣ�����ע������л�������ŵ����ʺ�ת������Ϸ�Ӧ�����жϿ��ܷ����ķ�Ӧ���ر����л�������ŵ����ʣ�Ϊ��������Ŀ�Ĺؼ���

��ϰ��ϵ�д�
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ
 ��2012?���Ķ�ģ��ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������
��2012?���Ķ�ģ��ij��Һ�п��ܺ���H+��Na+��NH4+��Mg2+��Fe3+��Al3+��SO42-��CO32-�����ӣ��������Һ�м���һ�����ʵ���Ũ�ȵ�NaOH��Һʱ���������ɳ��������ʵ�����NaOH��Һ������仯��ͼ����ͼ��ʾ������˵����ȷ���ǣ�������