��Ŀ����
���и���Һ�У���������ʵ���Ũ�ȹ�ϵ����ȷ���ǣ�������
������A������������Դ������
B�����ݵ���غ������
B�����������غ������
D����Ũ����笠�������ͬ�������Һ�У�������ˮ��̶��ж�笠�����Ũ�ȣ�
B�����ݵ���غ������
B�����������غ������
D����Ũ����笠�������ͬ�������Һ�У�������ˮ��̶��ж�笠�����Ũ�ȣ�
����⣺A��笠�������Դ��һˮ�ϰ����룬������������Դ��һˮ�ϰ���ˮ�ĵ��룬����c��NH4+����c��OH-������A��ȷ��
B�����ݵ���غ�֪��c��NH4+��+c��H+���Tc��Cl-��+c��OH-������B��ȷ��
C�����������غ��c��HCO3-��+c��CO3-��+c��H2CO3��=0.1 mol?L-1����C��ȷ��
D����Ũ����笠�������ͬ���⼸�������Һ�У�����������ӵ����������������笠�����ˮ�⣬��������Ӻ�笠�������ٽ�ˮ�⣬����笠�����Ũ�ȴ�С˳����CH3COONH4��NH4Cl��NH4HSO4����D����
��ѡD��
B�����ݵ���غ�֪��c��NH4+��+c��H+���Tc��Cl-��+c��OH-������B��ȷ��
C�����������غ��c��HCO3-��+c��CO3-��+c��H2CO3��=0.1 mol?L-1����C��ȷ��
D����Ũ����笠�������ͬ���⼸�������Һ�У�����������ӵ����������������笠�����ˮ�⣬��������Ӻ�笠�������ٽ�ˮ�⣬����笠�����Ũ�ȴ�С˳����CH3COONH4��NH4Cl��NH4HSO4����D����
��ѡD��
���������⿼��������Ũ�ȴ�С�ıȽϣ��Ѷ��еȣ��״�ѡ����D��ע���������ˮ��̶ȣ�Ϊ�״��㣮

��ϰ��ϵ�д�
�����Ŀ
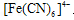 ��Ӧ������ɫ��������K4[Fe(CN)6]��Һ����Fe3+�������ȱ���KSCN���ߡ��������Ϣ�������������ʵ����֤���룺
��Ӧ������ɫ��������K4[Fe(CN)6]��Һ����Fe3+�������ȱ���KSCN���ߡ��������Ϣ�������������ʵ����֤���룺