��Ŀ����
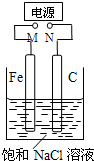 ��ͼ������Fe����ʯī������1L����NaCl��Һ�У�����˵����ȷ���ǣ�������
��ͼ������Fe����ʯī������1L����NaCl��Һ�У�����˵����ȷ���ǣ�������������M�Ӹ�����N����������������2Cl--2e-=Cl2������������2H2O+2e-=2OH-+H2����M�ӵ�Դ������N�ӵ�Դ��������C�缫����Cu�缫����������Fe-2e-=Fe2+����������Cu2++2e-=Cu���Դ˽����⣮
����⣺A��M�Ӹ�����N����������������2Cl--2e-=Cl2������������2H2O+2e-=2OH-+H2����������������������Ϊ22.4L����״����ʱ����������ʵ���Ϊ1mol������������0.5molH2��ͬʱ����1molNaOH����A��ȷ��
B��M�Ӹ�����N��������C�缫����2Cl--2e-=Cl2���������̪����죬��B����
C��M�Ӹ�����N�������������ձ�����Һ����1L CuSO4��Һ����������2Cl--2e-=Cl2������������Cu2++2e-=Cu��û��������ͭ�������ɣ���C����
D��M�ӵ�Դ������N�ӵ�Դ��������C�缫����Cu�缫����������Fe-2e-=Fe2+����������Cu2++2e-=Cu������ʵ�������϶�ͭ����D����
��ѡA��
B��M�Ӹ�����N��������C�缫����2Cl--2e-=Cl2���������̪����죬��B����
C��M�Ӹ�����N�������������ձ�����Һ����1L CuSO4��Һ����������2Cl--2e-=Cl2������������Cu2++2e-=Cu��û��������ͭ�������ɣ���C����
D��M�ӵ�Դ������N�ӵ�Դ��������C�缫����Cu�缫����������Fe-2e-=Fe2+����������Cu2++2e-=Cu������ʵ�������϶�ͭ����D����
��ѡA��
���������⿼����صĹ���ԭ����ע��缫��Ӧ���жϣ��������ӷŵ�˳��Ϊ������Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�
�����Ŀ
��ͼ������Fe����ʯī������1 L����NaCl��Һ�С�����˵����ȷ����
| A��M�Ӹ�����N��������������������������Ϊ22.4 L(��״��)ʱ������1 mol NaOH |
| B��M�Ӹ�����N������������Һ�е��˷�̪��Һ��C�缫��Χ��Һ��� |
| C��M�Ӹ�����N�������������ձ�����Һ����1 L CuSO4��Һ����Ӧһ��ʱ����ձ��в�����ɫ���� |
| D��M�ӵ�Դ������N�ӵ�Դ��������C�缫����Cu�缫���������Һ����CuSO4��Һ�����ʵ�������϶�ͭ |
 ��ͼ������Fe����ʯī������1L ����NaCl��Һ�У�����˵����ȷ���ǣ�������
��ͼ������Fe����ʯī������1L ����NaCl��Һ�У�����˵����ȷ���ǣ�������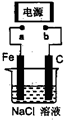 ��2009?���ڶ�ģ����ͼ������Fe����ʯī������1L 1.0mol/L��NaCl��Һ�У�����˵����ȷ���ǣ�������
��2009?���ڶ�ģ����ͼ������Fe����ʯī������1L 1.0mol/L��NaCl��Һ�У�����˵����ȷ���ǣ�������