��Ŀ����
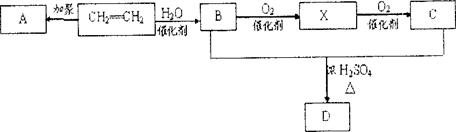
�߷���G�dz��úϳɲ��ϣ���ͨ������·�ߺϳɡ���֪F������ͼ������ʺɱ�Ϊ86������C��H��O��ԭ�Ӹ���֮��Ϊ2��3��1��F������������ˮ�������̼ԭ������ͬ�������л���D��E��
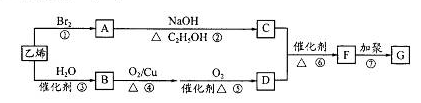
��ش��������⣺
��1��B�ķ���ʽΪ ��F���Ӻ��������ŵ�����Ϊ ��
��2��G�Ľṹ��ʽΪ ��
��3����Ӧ�١��ķ�Ӧ���;��� ��
��4����Ӧ�ڵĻ�ѧ����ʽΪ ��
��5����֪��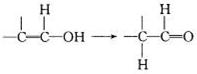 ����E�Ľṹ��ʽΪ ��
����E�Ľṹ��ʽΪ ��
��6����F��ͬ���칹���У��˴Ź��������������壬����������֮��Ϊ1��1��1������д������һ���������������Ľṹ��ʽ ��

��ش��������⣺
��1��B�ķ���ʽΪ ��F���Ӻ��������ŵ�����Ϊ ��
��2��G�Ľṹ��ʽΪ ��
��3����Ӧ�١��ķ�Ӧ���;��� ��
��4����Ӧ�ڵĻ�ѧ����ʽΪ ��
��5����֪��
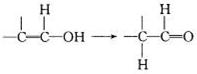 ����E�Ľṹ��ʽΪ ��
����E�Ľṹ��ʽΪ ����6����F��ͬ���칹���У��˴Ź��������������壬����������֮��Ϊ1��1��1������д������һ���������������Ľṹ��ʽ ��
(1)C2H6O(1��) ����(1��)
(2)
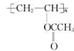 (1��)
(1��) (3)�ӳɷ�Ӧ(1��)
(4)CH2BrCH2Br+2NaOH
 HC��CH+2NaBr+2H2O(2��)
HC��CH+2NaBr+2H2O(2��)(5)CH3CHO(1��)
(6)
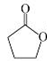 ��
��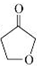 ��
��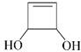 (2��)
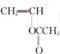
(2��)�������������F�����ʱ�Ϊ86��Mr=86��������n(C)��n(H)��n(O)=2��3��1���Ƴ�F�ķ���ʽΪ C4H6O2������·�ߢ�CH2=CH2��Br2�ӳɵõ�A��CH2BrCH2Br���پ�������ȥ�õ�C��HC��CH������·�ߢ�CH2=CH2��H2O�ӳɵõ�B��C2H5OH�����ܵõ�CH3CHO�پ����ݵõ�D��CH3COOH��F�ķ���ʽΪ C4H6O2��������������ˮ�������̼ԭ������ͬ�������л���D��CH3COOH��E��C4H6O2+H2O��CH3COOH+E�����Եó�E�ķ���ʽΪC2H4O����������F����ˮ��õ����ᣬ˵��������������Ϸ���ʽ�ó�����һ������������һ�������Ͷȣ����Է����Ӿ۷�Ӧ��˵������һ��̼̼˫������D��CH3COOH��E��C2H4O�����õ�F��������F����һ̼̼˫����˵����˫��ֻ��������E������E�л�����һ����OH��˵��������CH2=CHOH�����Կɵó�F�ĽṹΪ
 �����ɵó�G�ĽṹʽΪ
�����ɵó�G�ĽṹʽΪ �������������������εó�
�������������������εó�(1)C2H6O ���� (2)
 (3)�ӳɷ�Ӧ
(3)�ӳɷ�Ӧ(4)CH2BrCH2Br+2NaOH
 HC��CH+2NaBr+2H2O һ��Brԭ�Ӵ���һ��HBr���ӣ�����һ�������Ͷȣ���������ȥ2��HBr���ӣ�����2�������Ͷȣ��ԡ���ʽ���ڡ�
HC��CH+2NaBr+2H2O һ��Brԭ�Ӵ���һ��HBr���ӣ�����һ�������Ͷȣ���������ȥ2��HBr���ӣ�����2�������Ͷȣ��ԡ���ʽ���ڡ�(5)CH3CHO��������Ϣ�ɵ�CH2=CHOH��CH3CHO��
(6) F�ķ���ʽΪ C4H6O2�������������Ͷȡ�����������˫������״��������һ��˫����һ���������������������ݺ˴Ź�������������Hԭ�ӣ����Ҹ�����1:1:1�������������״�Ļ��Ͳ�����Hԭ�Ӹ�����1:1:1��������Ҫ����һ���������һ��˫����̼̼˫��������̼��˫�����ۺ��ƶϿɵó�
 ��
�� ��
�� ���ֽṹ
���ֽṹ
��ϰ��ϵ�д�
�����Ŀ

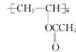
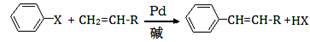 ��XΪ±ԭ�ӣ�RΪȡ������
��XΪ±ԭ�ӣ�RΪȡ������
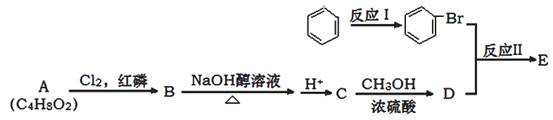
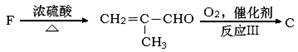
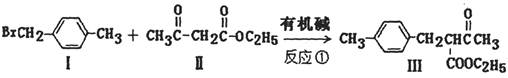
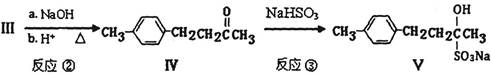
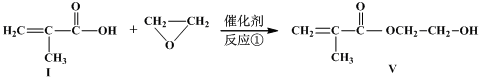
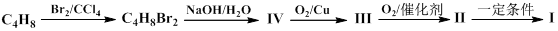
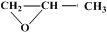 �������Ʒ�Ӧ�ٵķ�Ӧ����д������һ�ֲ���Ľṹ��ʽ ��
�������Ʒ�Ӧ�ٵķ�Ӧ����д������һ�ֲ���Ľṹ��ʽ ��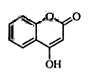
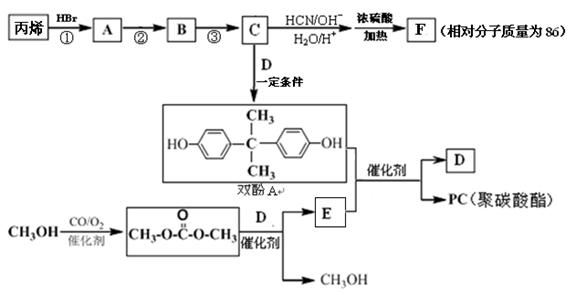
 ��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳɣ�
��һ��ҽҩ�м��壬�������Ʊ�����Ѫҩ����ͨ������·�ߺϳɣ�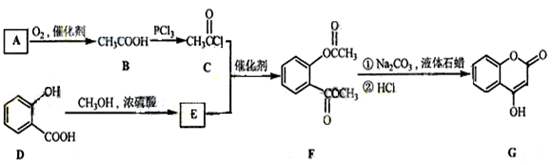
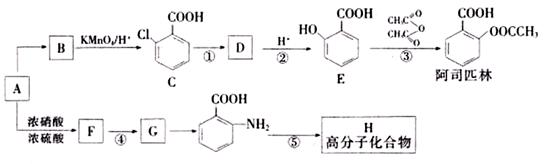
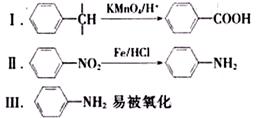
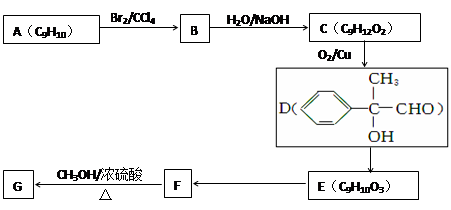
 B�ķ�Ӧ����(ѡ��ȡ����Ӧ��ӳɷ�Ӧ) ��
B�ķ�Ӧ����(ѡ��ȡ����Ӧ��ӳɷ�Ӧ) ��