��Ŀ����
��8�֣�������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼ��װ���Ʊ�����������

��1����̲IJ��õ�ʵ��װ�ò�ͬ����װ���в��������θ���ܣ��������ǣ� ��
��2��Ϊ��֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý���������4��ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
|
ʵ���� |
�Թܢ����Լ� |
�Թܢ��� �Լ� |
���� ���/cm |
|
A |
2 mL�Ҵ���1 mL���ᡢ 1mL18mol��L��1 Ũ���� |
����Na2CO3 ��Һ |
3.0 |
|
B |
2 mL�Ҵ���1 mL���� |
0.1 |
|
|
C |
2 mL�Ҵ���1 mL���ᡢ 3 mL 2mol��L��1 H2SO4 |
0.6 |
|
|
D |
2 mL�Ҵ���1 mL���ᡢ���� |
0.6 |
�� ʵ��D��Ŀ������ʵ��C����գ�֤��H+��������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ���______mL��_____mol��L��1 ��
�� ����ʵ�� ����ʵ���ţ������ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʡ�
��3������������90g���Ҵ�138g����������Ӧ�õ�80g�����������Լ���÷�Ӧ�IJ���Ϊ______________��
(��8��)��1�������ͷ�ֹ������2�֣���
��2����3 4 ����1�֣� ��A C��2��,��ѡ���÷֣�©ѡ��1�֣� ��3�� 60.6%
����������1��Ӧ��������Ҵ����Ǻ�ˮ���ܵģ����Կ����ֹ���������ã�ͬʱҲ���������á�
��2���ټ�Ȼ�Ƕ���ʵ�飬�������ӵ�Ũ��Ӧ����ͬ�ģ����������Ũ����4mol/L�������3ml��
�ڸ���A��C�����ɵ������������������жϣ�ŨH2SO4����ˮ����������������IJ��ʡ�
��3�����ݷ�ӦʽCH3COOH��CH3CH2OH CH3COOCH2CH3��H2O��֪��90g����Ӧ�������Ҵ�69g������������Ӧ����������������132g���Ҵ�������80��132��100����60.6����
CH3COOCH2CH3��H2O��֪��90g����Ӧ�������Ҵ�69g������������Ӧ����������������132g���Ҵ�������80��132��100����60.6����

 �����ҵ��ٿ���������������ϵ�д�
�����ҵ��ٿ���������������ϵ�д�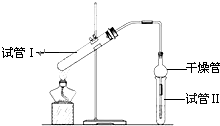 ������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ��������ͼ��װ���Ʊ�����������
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ��������ͼ��װ���Ʊ�������������1����̲IJ��õ�ʵ��װ�ò�ͬ����װ���в��������θ���ܣ��������ǣ�
��2��Ϊ��֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý���������4��ʵ�飮ʵ�鿪ʼ���þƾ���
��3min���ټ���ʹ֮����3min��ʵ�����������С�Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܢ����Լ� | �Թܢ��� �Լ� |
���� ���/cm |
| A | 2mL�Ҵ���1mL���ᡢ 1mL18mol?L-1 Ũ���� |
����Na2CO3 ��Һ |
3.0 |
| B | 2mL�Ҵ���1mL���� | 0.1 | |
| C | 2mL�Ҵ���1mL���ᡢ 3mL 2mol?L-1H2SO4 |
0.6 | |
| D | 2mL�Ҵ���1mL���ᡢ���� | 0.6 |
�ڷ���ʵ��
��3������������90g���Ҵ�138g����������Ӧ�õ�80g�����������Լ���÷�Ӧ�IJ���Ϊ
��8�֣�
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼ��װ���Ʊ�����������
��1����̲IJ��õ�ʵ��װ�ò�ͬ����װ���в��������θ���ܣ�����
���ǣ� ��
��2��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý���������4��ʵ�顣ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ�����������С�Թ�II�ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ���� | �Թܢ����Լ� | �Թܢ����Լ� | ����л���ĺ��/cm |
| A | 3 mL�Ҵ���2 mL���ᡢ1mL18mol��L��1 Ũ���� | ����Na2CO3��Һ | 5��0 |
| B | 3 mL�Ҵ���2 mL���� | 0��1 | |
| C | 3 mL�Ҵ���2 mL���ᡢ6 mL 3mol��L��1 H2SO4 | 1��2 | |
| D | 3 mL�Ҵ���2 mL���ᡢ���� | 1��2 |
�� ʵ��D��Ŀ������ʵ��C����գ�֤��H+��������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ���__________mL��__________mol��L��1 ��
�� ����ʵ�� ����ʵ���ţ������ݣ������Ʋ��ŨH2SO4����ˮ����������������IJ��ʡ�
�� ����������������������IJ��ʣ���ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ����ܵ�ԭ���� ��
��10�֣�
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ�������������Ҵ���Ӧ�Ʊ�����������Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�� ��ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
��ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�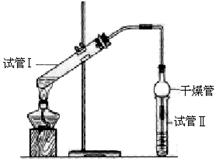
| ʵ�� ��� | �Թܢ��е��Լ� | �Թܢ����Լ� | ����л���ĺ��/cm |
| A | 2mL�Ҵ���2mL���ᡢ1mL 18mol��L-1Ũ���� | | 5.0 |
| B | 3mL�Ҵ���2mL���� | 0.1 | |
| C | 3mL�Ҵ���2mL���ᡢ6mL 3mol��L-1���� | 1.2 | |
| D | 3mL�Ҵ���2mL���ᡢ���� | 1.2 |
��ʹ�ø���ܵ�Ŀ���� ��
��2�������Ƚ�ʵ�� ����ʵ���ţ������ݣ������Ʋ��Ũ�������ˮ����������������IJ��ʡ�Ũ�������ˮ���ܹ���������������ʵ�ԭ���� ��
��3��ʵ��D��Ŀ������ʵ��C����գ�֤��H����������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ��� mL�� mol��L-1��
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ��������ˮ�Ҵ��������Ʊ����������Ļ�ѧ����ʽ���£�
CH3COOH��C2H5OH CH3COOC2H5��H2O
CH3COOC2H5��H2O
��1���÷�Ӧ��ƽ�ⳣ������ʽK������ ����
��2��Ϊ֤��Ũ�����ڸ÷�Ӧ�����˴�������ˮ�������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�飬ʵ�鿪ʼ���þƾ�����3min���ټ���ʹ֮����3min��ʵ������������Թܢ��ٲ��л���ĺ�ȣ�ʵ���¼���£�
| ʵ�� ��� | �Թܢ��е��Լ� | �Թܢ����Լ� | ����л���ĺ��/cm |
| A | 2mL�Ҵ���2mL���ᡢ1mL 18mol/LŨ���� | ����̼������Һ | 5.0 |
| B | 3mL�Ҵ���2mL���� | 0.1 | |
| C | 3mL�Ҵ���2mL���ᡢ6mL 3mol/L���� | 1.2 | |
| D | 3mL�Ҵ���2mL���ᡢ���� | 1.2 |
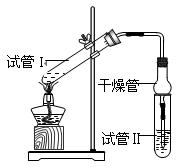
��ʵ��D��Ŀ������ʵ��C����գ�֤��H����������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ��� mL�� mol/L��
�ڷ���ʵ�� ����ʵ���ţ������ݣ������Ʋ��Ũ�������ˮ����������������IJ��ʡ�Ũ�������ˮ���ܹ���������������ʵ�ԭ���� ��
�ۼ���������������������IJ��ʣ���ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ����ܵ�ԭ���� ��
 ������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼ��װ���Ʊ�����������
������������Ҫ���л��ϳ��м��壬�㷺Ӧ���ڻ�ѧ��ҵ��ʵ����������ͼ��װ���Ʊ�����������