��Ŀ����
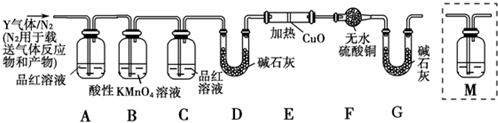
13��ij����С���ͬѧ����ʵ������п��Ũ���ᷴӦ��ʵ��ʱ����ͬѧ��Ϊ�����������SO2�⣬�����ܲ�����������ͬѧΪ����֤�����ж��Ƿ���ȷ���������ͼ��ʾ��ʵ��װ�ã����У�װ��B��ʢ��Ũ���ᣬװ��C�з��ú�ɫCuO��ĩ��װ��D�з��õ�����ˮ����ͭ��ĩ��п��Ũ���Ṳ��ʱ����������ΪX���Ҹ�װ����ȥ�����Իش�
��1��A�м�����Լ�������Ʒ����Һ�������Ǽ�������X���Ƿ���SO2��E�м�����Լ��Ǽ�ʯ�ң������Ƿ�ֹ�����е�ˮ��������D�У����ż��飻װ��A��B֮������Ը��������Һ�������dz�ȥ����X�е�SO2��
��2����ͬѧ��Ϊ�����ܲ���������������п��Ũ���ᷴӦʱ��Ũ������ϡ��п��ϡ���ᷴӦ������������
��3��������Ӧ�����ɶ�������Ļ�ѧ����ʽΪZn+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ZnSO4+SO2��+2H2O��
��4�����ȥ��װ��B�����ܷ����D�е������ж�����X�������������ܣ���ܡ����ܡ���ԭ���ǣ���Ϊ��������п��ܺ���H2O�����H2�ļ��飮
��5������֤������X�к���������ʵ�������ǣ�
C�к�ɫ��ĩ��CuO����ɺ�ɫ���������ʣ�
D�а�ɫ��ĩ�����ɫ���壮
���� Ϊ����֤���ɵ��������ж��������������װ��A����֤����������ڵ�װ�ã�ѡƷ����Һ������֤��ͨ�����������Һ��ȥ��������ͨ��װ��B�е�Ũ�����ȥˮ��������������������ͭ��Ӧ����ͭ��ˮ��������������װ��D�� ����ˮ����ͭ����ˮ�����ɣ�Ϊ��������е�ˮ����Ӱ��Dװ����ˮ�ļ��飬װ��E����Ҫ�ü�ʯ�ң��Դ˽����⣮
��� �⣺��1��Aװ�õ������Ǽ����������Ĵ��ڣ�����������Ư��Ʒ�죬����ͨ��Ʒ����Һ��ɫ����SO2�Ĵ��ڣ�������Ʒ����ɫ����ʯ�ҵ���Ҫ�ɷ�Ϊ�����ƺ��������ƣ�������ˮ�Ͷ�����̼��Ϊ��ֹ�����е�ˮ��������D�У�A��Bװ��֮������Ը��������Һ���ڳ�ȥ����X�еĶ����������壬
�ʴ�Ϊ��Ʒ����Һ����������X���Ƿ���SO2����ֹ�����е�ˮ��������D�У����ż��飻��ȥ����X�е�SO2��
��2����Zn��ŨH2SO4 ��ӦʱŨH2SO4 Ũ����ϡ��Zn��ϡH2SO4 ��ӦZn+H2SO4=ZnSO4+H2��������H2��
�ʴ�Ϊ��п��Ũ���ᷴӦʱ��Ũ������ϡ��п��ϡ���ᷴӦ������������
��3���������ǿ�����ԣ�����п���л�ԭ�ԣ�Zn��ŨH2SO4��Ӧ��������п�����������ˮ����Ӧ�ķ���ʽΪ��Zn+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ ZnSO4+SO2��+2H2O��
�ʴ�Ϊ��Zn+2H2SO4 ��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$ ZnSO4+SO2��+2H2O��
��4��п�����ᷴӦ���ɵ������бغ���ˮ�����ȥ��װ��B����Ϊ��������к��е�H2O�����H2�ļ��飬
�ʴ�Ϊ�����ܣ���Ϊ��������п��ܺ���H2O�����H2�ļ��飻
��5��Ϊ�˼��������Ĵ��ڣ�����������ͭ�ڼ��������·�Ӧ����ͭ��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��H2+CuO$\frac{\underline{\;\;��\;\;}}{\;}$Cu+H2O��ɫCuO��ɺ�ɫ��Cu��������ˮ����ͭ���������������IJ���ˮ��ˮ����ˮ����ͭ��Ӧ������ɫ����ˮ������ͭ�����Կ���֤������X�к���H2��ʵ��������C�к�ɫCuO��ɺ�ɫ��Cu��D�а�ɫ��ĩ�����ɫ��
�ʴ�Ϊ����ɫ��ĩ��CuO����ɺ�ɫ���������ʣ���ɫ��ĩ�����ɫ���壮
���� ���⿼�����ʵ�����ʵ�飬Ϊ��Ƶ���㣬������ѧ���ķ���������ʵ�������Ŀ��飬��Ŀ�Ѷ��еȣ�ע������Ũ��������ʣ������������������ʡ����鷽�����������Ⱥ�˳���ǽ��Ĺؼ���

 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д� ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д�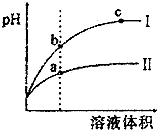 2011��8�£����ý�屨������ɽ���ϳ´����ɴ����Ҷ��ɣ������DZ����ᣮר�ҽ��ܣ�ֻҪ�Ƿ��Ϲ��ұ��Ĺ��Ҵף��ǿ��Է���ʳ�õģ����й��ڴ����˵������ȷ���ǣ�������
2011��8�£����ý�屨������ɽ���ϳ´����ɴ����Ҷ��ɣ������DZ����ᣮר�ҽ��ܣ�ֻҪ�Ƿ��Ϲ��ұ��Ĺ��Ҵף��ǿ��Է���ʳ�õģ����й��ڴ����˵������ȷ���ǣ�������| A�� | 0.01mol•L-l�Ĵ�����Һ��Ph=2 | |
| B�� | ��ij�¶��µĴ�����Һ��ͨ��HCl���壬����ĵ��볣��Ka������ | |
| C�� | ��pH�����������ϡ�ͺ�pH�ı仯��ͼ��ʾ�������ߢ��ʾ���������ϡ��ͼ����Һ��� | |
| D�� | ͼ�У�a��b��c�����ʾ����Һ�ĵ�����ǿ����ϵΪa��b��c |
| A�� | A-B֮ǰ | B�� | B-C�� | C�� | C-D�� | D�� | D-E�� |
| A�� |  | B�� |  | C�� |  | D�� |  |

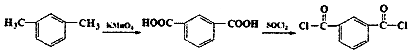
 +2
+2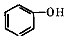 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ +H2O
+H2O +SOCl2��
+SOCl2�� +SO2+HCl
+SO2+HCl +HCl
+HCl ��
��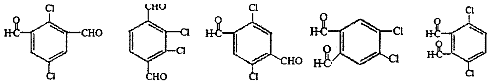 ���������֣���
���������֣��� ��
�� X��Y��Z��W��Ϊ���嵥�ʣ�A��B��C��Ϊ��ɫ���廯���D��E��Ϊ��ɫ���壬F��ͨ�������Ϊ��ɫ��ζ��Һ�壬���������µ�ת����ϵ��
X��Y��Z��W��Ϊ���嵥�ʣ�A��B��C��Ϊ��ɫ���廯���D��E��Ϊ��ɫ���壬F��ͨ�������Ϊ��ɫ��ζ��Һ�壬���������µ�ת����ϵ�� ��F�еĻ�ѧ������Ϊ���ۼ���
��F�еĻ�ѧ������Ϊ���ۼ���

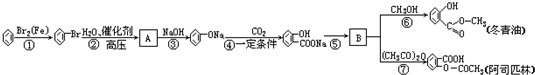
 ��
�� ��
��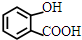 +NaHCO3��
+NaHCO3��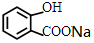 +CO2��+H2O��
+CO2��+H2O��