��Ŀ����
����һ�仰������������ֵ��ǰ��ΪM������ΪN��M��N�Ĺ�ϵ��A��B��C��D��ѡ��
A��M��NB��M��NC��M=ND�����Ƚ�
��1����ͬ�¶��£�1L1mol/L��NH4Cl��Һ��2L0.5mol?L-1NH4Cl��Һ������Һ��NH4+�ĸ�����______��
��2����ͬ�¶��£�pHֵΪ12���ռ���Һ��CH3COONa��Һ������Һ��ˮ�ĵ���̶ȣ�______��
��3�����������ݵ�Ũ�ȵĴ�����Һ�����ڶ��������¶ȣ�����Һ��c��HCO3-����______��
��4����pHֵΪ2�Ĵ�������ᶼϡ����ͬ��������ϡ��Һ��pHֵ��______��
A��M��NB��M��NC��M=ND�����Ƚ�
��1����ͬ�¶��£�1L1mol/L��NH4Cl��Һ��2L0.5mol?L-1NH4Cl��Һ������Һ��NH4+�ĸ�����______��
��2����ͬ�¶��£�pHֵΪ12���ռ���Һ��CH3COONa��Һ������Һ��ˮ�ĵ���̶ȣ�______��
��3�����������ݵ�Ũ�ȵĴ�����Һ�����ڶ��������¶ȣ�����Һ��c��HCO3-����______��
��4����pHֵΪ2�Ĵ�������ᶼϡ����ͬ��������ϡ��Һ��pHֵ��______��
��1���Ȼ���У�笠�����ˮ�⣬笠�����Ũ��Խ��ˮ��̶�ԽС������ͬ�¶��£�1L 1mol/L ��NH4Cl��Һ�е�NH4+��������2L 0.5mol?L-1NH4Cl��Һ��NH4+�ĸ������ʴ�Ϊ��A��
��2��pHֵΪ12���ռ���Һ��ˮ�������������Ũ��Ϊ10-12mol/L��pHֵΪ12��CH3COONa��Һ��ˮ�������������Ũ��Ϊ10-2mol/L��������ͬ�¶��£�pHֵΪ12���ռ���Һ��ˮ�ĵ����С��pHֵΪ12��CH3COONa��Һ��ˮ�ĵ���ȣ��ʴ�Ϊ��B��
��3��̼������Һ�У����´ٽ�̼�����ˮ�⣬���������¶�ʱ��̼�������Ũ�����ʴ�Ϊ��B��
��4�����������ᣬϡ�ʹٽ����룬������ǿ�ᣬϡ����Ũ�ȼ�С�����Խ�pHֵΪ2�Ĵ�������ᶼϡ����ͬ��������ϡ��Һ��pHֵ�Ǵ����С������ģ��ʴ�Ϊ��B��
��2��pHֵΪ12���ռ���Һ��ˮ�������������Ũ��Ϊ10-12mol/L��pHֵΪ12��CH3COONa��Һ��ˮ�������������Ũ��Ϊ10-2mol/L��������ͬ�¶��£�pHֵΪ12���ռ���Һ��ˮ�ĵ����С��pHֵΪ12��CH3COONa��Һ��ˮ�ĵ���ȣ��ʴ�Ϊ��B��
��3��̼������Һ�У����´ٽ�̼�����ˮ�⣬���������¶�ʱ��̼�������Ũ�����ʴ�Ϊ��B��
��4�����������ᣬϡ�ʹٽ����룬������ǿ�ᣬϡ����Ũ�ȼ�С�����Խ�pHֵΪ2�Ĵ�������ᶼϡ����ͬ��������ϡ��Һ��pHֵ�Ǵ����С������ģ��ʴ�Ϊ��B��

��ϰ��ϵ�д�
�����Ŀ

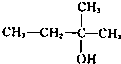
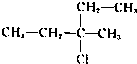
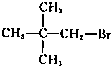
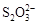

 ��2I�D��������ʵ��ܶȻ��������±���
��2I�D��������ʵ��ܶȻ��������±���