��Ŀ����
Ī�����������ε�һ�ָ��Σ��ɷ��ǣ�NH4��2Fe��SO4��2������һ����Ҫ�Ļ�ѧ�Լ��������·�Ӧ��
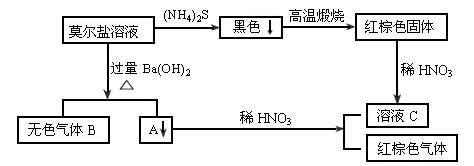
�������Ϲ�ϵ����Ҫ����գ�
��1������B�ĵ���ʽΪ ������ɫ����F�����ºͽ�������Ӧ���ɽ���G�Ļ�ѧ����ʽΪ ��
��2������A��ϡHNO3��Ӧ�����ӷ���ʽΪ ��
��3����16.8g����Gǡ����ȫ��Ӧ��ϡHNO3�ܽ����ͭ�����ʵ���x�ķ�ΧΪ ��
��4��NH4HSO4�������һ����ʽ�Σ���0.01mol/L NH4HSO4��Һ�еμ�NaOH��Һ�����ԣ���c��Na+�� c��SO42-����c��NH4+�� c��SO42-�������������������=����
��5���뽫������Һ��NH4+Ũ���ɴ�С����
��0.02mol/L NH4HSO4 ��Һ
��0.01mol/L��NH4��2Fe��SO4��2��Һ
��0.01mol/L��NH4��2 CO3��Һ��

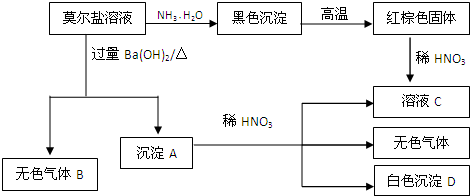
�������Ϲ�ϵ����Ҫ����գ�
��1������B�ĵ���ʽΪ
��2������A��ϡHNO3��Ӧ�����ӷ���ʽΪ
��3����16.8g����Gǡ����ȫ��Ӧ��ϡHNO3�ܽ����ͭ�����ʵ���x�ķ�ΧΪ
��4��NH4HSO4�������һ����ʽ�Σ���0.01mol/L NH4HSO4��Һ�еμ�NaOH��Һ�����ԣ���c��Na+��
��5���뽫������Һ��NH4+Ũ���ɴ�С����
��0.02mol/L NH4HSO4 ��Һ
��0.01mol/L��NH4��2Fe��SO4��2��Һ
��0.01mol/L��NH4��2 CO3��Һ��
������Ī�����������ε�һ�ָ��Σ��ɷ��ǣ�NH4��2Fe��SO4��2����ת����ϵ��֪������AΪFe��0H��2��BaSO4����ɫ����BΪNH3����FeԪ���غ㼰��ɫ�����֪����ɫ����ΪFe2O3����ҺCΪFe��NO3��3����ɫ����DΪBaSO4��Ȼ�������ʵ����ʼ���ѧ���������
����⣺Ī�����������ε�һ�ָ��Σ��ɷ��ǣ�NH4��2Fe��SO4��2����ת����ϵ��֪������AΪFe��0H��2��BaSO4����ɫ����BΪNH3����FeԪ���غ㼰��ɫ�����֪����ɫ����ΪFe2O3����ҺCΪFe��NO3��3����ɫ����DΪBaSO4��
��1��BΪNH3�������ʽΪ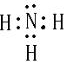 ������ɫ����F�����ºͽ�������Ӧ���ɽ���G�Ļ�ѧ����ʽΪ2Al+Fe2O3
������ɫ����F�����ºͽ�������Ӧ���ɽ���G�Ļ�ѧ����ʽΪ2Al+Fe2O3
Al2O3+2Fe��
�ʴ�Ϊ��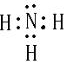 ��2Al+Fe2O3
��2Al+Fe2O3
Al2O3+2Fe��
��2������A��ϡHNO3��Ӧ�����ӷ���ʽΪFe��0H��2+10H++NO3-=3Fe3++NO��+4H2O���ʴ�Ϊ��Fe��0H��2+10H++NO3-=3Fe3++NO��+4H2O��
��3��GΪFe��n��Fe��=
=0.3mol�������ᷴӦ��������������������������ǡ����ȫ��Ӧ��ϡHNO3Ϊ0.8mol��1.2mol����3Cu��8HNO3��֪���ܽ��Cu��СΪ0.3mol�����Ϊ
=0.45mol������0.3��x��0.45���ʴ�Ϊ��0.3��x��0.45��
��4����0.01mol/L NH4HSO4��Һ�еμ�NaOH��Һ�����ԣ���1��1��Ӧ���Լ��ԣ���NaOH��������c��Na+����c��SO42-����������ʱ��Һ������Ϊ�����ƣ�����李�һˮ�ϰ���c��Na+��+c��NH4+��=2c��SO42-����c��Na+����c��SO42-������c��NH4+������SO42-�����ʴ�Ϊ����������
��5��������ٽ�ˮ�⣬����Ũ����С���٢ھ������ˮ�⣬����������Ũ�ȴ����Ƴ̶ȴ����������Ũ���������NH4+Ũ���ɴ�С����Ϊ�٣��ڣ��ۣ��ʴ�Ϊ���٣��ڣ��ۣ�
��1��BΪNH3�������ʽΪ
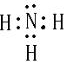 ������ɫ����F�����ºͽ�������Ӧ���ɽ���G�Ļ�ѧ����ʽΪ2Al+Fe2O3
������ɫ����F�����ºͽ�������Ӧ���ɽ���G�Ļ�ѧ����ʽΪ2Al+Fe2O3
| ||
�ʴ�Ϊ��
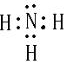 ��2Al+Fe2O3
��2Al+Fe2O3
| ||
��2������A��ϡHNO3��Ӧ�����ӷ���ʽΪFe��0H��2+10H++NO3-=3Fe3++NO��+4H2O���ʴ�Ϊ��Fe��0H��2+10H++NO3-=3Fe3++NO��+4H2O��
��3��GΪFe��n��Fe��=
| 16.8g |
| 56g/mol |
| 3��1.2mol |
| 8 |
��4����0.01mol/L NH4HSO4��Һ�еμ�NaOH��Һ�����ԣ���1��1��Ӧ���Լ��ԣ���NaOH��������c��Na+����c��SO42-����������ʱ��Һ������Ϊ�����ƣ�����李�һˮ�ϰ���c��Na+��+c��NH4+��=2c��SO42-����c��Na+����c��SO42-������c��NH4+������SO42-�����ʴ�Ϊ����������
��5��������ٽ�ˮ�⣬����Ũ����С���٢ھ������ˮ�⣬����������Ũ�ȴ����Ƴ̶ȴ����������Ũ���������NH4+Ũ���ɴ�С����Ϊ�٣��ڣ��ۣ��ʴ�Ϊ���٣��ڣ��ۣ�
���������⿼��������ƶϣ�Ϊ�߿��������ͣ��ۺ��Խ�ǿ�����������仯��������ʡ������Ρ����ȷ�Ӧ����������ʵ�Ϊ���Ĺؼ������ؼ��㼰����ˮ�⡢����Ũ�ȴ�С�ıȽϵȷ�Ӧԭ���Ŀ��飬��Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ