��Ŀ����
�������˻ᡰ���ơ����ȼ���DZ��飨C3H8��������������˻���ȼ���DZ�ϩ��C3H6������֪��C3H8(g)+5O2(g)����3CO2(g)+4H2O(l)+2220.0kJ
C3H6(g)+4.5O2(g)����3CO2(g)+3H2O(l)+2049kJ
����˵����ȷ���ǣ� ����
C3H6(g)+4.5O2(g)����3CO2(g)+3H2O(l)+2049kJ
����˵����ȷ���ǣ� ����
| A��1molC3H8(g)��5molO2(g)��Ӧ����3molCO2(g)��4molH2O(g)�ų���������2220.0kJ |
| B��1molC3H6��4.5molO2��Ӧ����3molCO2��3molH2O�ų���������2049.0kJ |
| C��������Ӵ�����������ڱ�ϩ���� |
| D������ת��Ϊ��ϩ�Ĺ�����һ�����ȹ��� |
C
A��ˮ��Һ̬�����壬�����������ʷų�������С��2220.0KJ��
B��û��˵�����ʵľۼ�״̬���ر���ˮ�ģ��ʲ����жϣ�
C������ų��������࣬��������
D����������������ڱ�ϩ�����Ƿ��ȹ���
B��û��˵�����ʵľۼ�״̬���ر���ˮ�ģ��ʲ����жϣ�
C������ų��������࣬��������
D����������������ڱ�ϩ�����Ƿ��ȹ���

��ϰ��ϵ�д�
 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
�����Ŀ
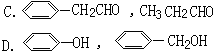
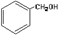 ���� �COH
���� �COH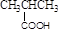 ���ᡡ �CCHO
���ᡡ �CCHO ȩ�ࡡ�CCHO
ȩ�ࡡ�CCHO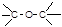

 ���ں͢� D���ں͢�
���ں͢� D���ں͢�