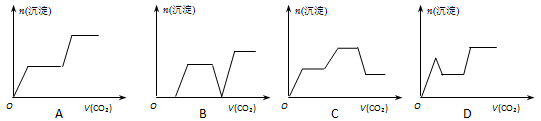
��Ŀ����
��14�֣�����A��B��C������ɫ�������ηֱ�����Na+��Ag+ ��Ba2+��Cl����NO3�� ��CO32���в�ͬ����������������ɡ���ʵ��A��Һ��B��Һ�����������ᷴӦ������A������ɫ������B�������ݡ���AΪ ��BΪ ����B��C����Һ��Ϸ�Ӧ�Ļ�ѧ����ʽΪ�� ��
������һ�������ĩ����CaCO3��Na2SO4��KNO3��BaCl2��CuSO4�е�����������ɣ�ȡ��Ʒ��������ʵ�飺

��ʵ������жϣ��ù����ĩ��һ�������� ��
������ɿ����� �� ��
������һ�������ĩ����CaCO3��Na2SO4��KNO3��BaCl2��CuSO4�е�����������ɣ�ȡ��Ʒ��������ʵ�飺

��ʵ������жϣ��ù����ĩ��һ�������� ��
������ɿ����� �� ��
��14�֣����� AgNO3 Na2CO3 BaCl2+Na2CO3=BaCO3 ��+2NaCl
����CuSO4 CaCO3��Na2SO4��KNO3 CaCO3��KNO3��BaCl2
����CuSO4 CaCO3��Na2SO4��KNO3 CaCO3��KNO3��BaCl2
�������������B��Һ�������ݣ�����B�к���CO32��������B��̼���ƣ�A��Һ������ɫ����������A�к��������ӣ���AӦ����������������C������������B��C��Ӧ�ķ���ʽ��BaCl2+Na2CO3=BaCO3 ��+2NaCl��
����������ˮ����ɫ��Һ�Ͱ�ɫ����������һ��û������ͭ�����ڰ�ɫ����������������������ɫ�������ɫ��Һ������ɫ�����������ᱵ������̼��ƣ��������ƺ��Ȼ�������ͬʱ���ڣ����ڸ�������������������ɵģ�����������ɿ�����CaCO3��Na2SO4��KNO3 ��CaCO3��KNO3��BaCl2��
�������������ʵļ���ʱ��Ҫ�������ʵ��������ʺ�������Ӧ��ѡ���ʵ����Լ��ͷ�����ȷ�۲췴Ӧ�е�������������ɫ�ı仯�����������ɺ��ܽ⡢����IJ�������ζ���������ɫ�ȣ������жϡ���������֤���ɡ�

��ϰ��ϵ�д�
�����Ŀ