��Ŀ����
14���������ƣ�NaClO2����Ҫ�����ġ���ֽҵ��Ư����Ҳ����ʳƷ������ˮ�����ȣ��������������ֽ⣮�������Ƶ�Ϊԭ���Ʊ��������ƵĹ����������£�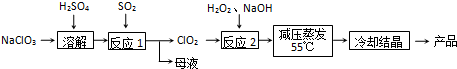
��1����ߡ���Ӧ1����Ӧ���ʵĴ�ʩ���ʵ����߷�Ӧ�¶ȡ���������ҺŨ�ȵȣ�
��2������Ӧ2���Ļ�ѧ����ʽΪH2O2+2ClO2+2NaOH=2NaClO2+2H2O+O2���÷�Ӧ����������ClO2��
��3����ȡ����ѹ�����������á���ѹ��������ԭ���dz�ѹ�����¶ȹ��ߣ������������ֽ⣮
��4���ӡ�ĸҺ���пɻ��յ���Ҫ������Na2SO4��
��5������ȴ�ᾧ�����ˡ���ϴ�ӣ���������ƣ������T�ɻ�ò�Ʒ��
���� NaClO3��ŨH2SO4�ڷ�Ӧ��I�����������Ӧ����ClO2��Na2SO4��ClO2�ڷ�Ӧ��II����˫��ˮ���������Ʒ�Ӧ�����������ƣ��ٵõ��侧�壬
��1��������Һ�еĻ�ѧ��Ӧ�������¶Ȼ���������������Ӧ���ʣ�
��2������ClԪ�ػ��ϼ۵ı仯�ж���������
��3����ѹ�����ڽϵ��¶��¿ɽ��У���ֹ�¶ȹ��߶��������ʷֽ⣻
��4���������ʵ������жϿ��ܷ����ķ�Ӧ���Դ˿��ж������
��5����ȴ�ᾧ��Ҫ��������壬Ӧ�ù��˵ķ�����
��� �⣺NaClO3��ŨH2SO4�ڷ�Ӧ��I�����������Ӧ����ClO2��Na2SO4��ClO2�ڷ�Ӧ��II����˫��ˮ���������Ʒ�Ӧ�����������ƣ��ٵõ��侧�壬
��1�����������Һ��ķ�Ӧ��Ϊ��߷�Ӧ���ʣ����ʵ����߷�Ӧ�¶ȣ���������ҺŨ�ȣ�����SO2������Һ�ĽӴ������
�ʴ�Ϊ���ʵ����߷�Ӧ�¶ȣ���������ҺŨ�ȣ�
��2�����������ԣ���������NaClO2Ŀ������ص��֪����Ӧ�ķ���ʽΪH2O2+2ClO2+2NaOH=2NaClO2+2H2O+O2����Ӧ��ClԪ�صĻ��ϼ۽��ͣ���ClO2Ϊ��������
�ʴ�Ϊ��2NaClO2+2H2O+O2��ClO2��
��3����ѹ�����ڽϵ��¶��¿ɽ��У���ֹ��ѹ�����¶ȹ��ߣ������������ֽ⣬
�ʴ�Ϊ����ѹ�����¶ȹ��ߣ������������ֽ⣻
��4����������������������������·���������ԭ��Ӧ����ClO2��Na2SO4����ĸҺ��Ӧ����Na2SO4��
�ʴ�Ϊ��Na2SO4��
��5����ȴ�ᾧ��Ҫ��������壬Ӧ�ù��ˡ�ϴ�ӡ�����ʴ�Ϊ�����ˣ�ϴ�ӣ�
���� ���⿼�����ʵ��Ʊ�ʵ��Ĺ�ҵ��ƣ���Ŀ�Ѷ��еȣ�����ע��������ʵ����ʣ��������غ�ĽǶ��Լ�������ԭ��Ӧ���ص��ж������Ϊ������Ĺؼ���Ҳ���״��㣮


�������ʵ��й��������£�
| ��ѧʽ | N2 | O2 | CO2 | NH3 | Cl2 |
| �۵㣨�棩 | -209.86 | -218.4 | -78.5 | -77.3 | -101 |
| �е㣨�棩 | -195.8 | -183 | -33.35 | -34.6 |
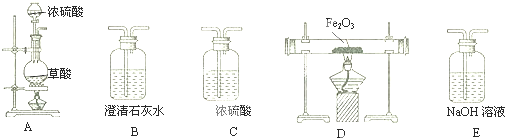
��1����˵�������Լ�ǰ����A��B��C�����������ԵIJ��������ȹرշ�Һ©���ϵĻ������رտ���N�Ļ�������M�ܿڲ���ʢ��ˮ��ˮ���У���A����Բ����ƿ�ȣ����ܿ�M��������ð����ֹͣ����M����Һ��������֤��A��B��C����װ�����������ã�
��2��A��ʢ�е���ɫ�����Լ�aӦ�Ǹ�����أ���Һ©���е�b�Լ���Ũ���ᣮ
��3��B��ʢ�е�Һ��CӦ�DZ���ʳ��ˮ��C�е�Һ��d��Ũ���ᣮ
��4��D�з�����Ӧ�Ļ�ѧ����ʽ��2HgO+2Cl2=Cl2O+HgO•HgCl2��
��5��E�еı���ƿ��ʢ��Һ̬�����c����Ӧ��Һ̬�������ڡ��ɱ���������ˮ������Һ̬����������Һ��������Һ�ȡ���ѡ��һ�֣�����E���ڹܵõ���Cl2O�п��ܺ��е�������Ҫ��Һ�ȣ�
��6��װ��A��B��C������ӷ�ʽ��D��E������ӷ�ʽ�����ԵIJ����������A��B��C�����齺�����ӣ�D��E�䲻���齺�����ӣ������ֲ�ͬ�����ӷ�ʽ����Ҫ������Cl2O���л����ױ�ը��
| ʵ�� ��� | HA�����ʵ��� Ũ�ȣ�mol•L-1�� | NaOH�����ʵ��� Ũ�ȣ�mol•L-1�� | ��Ϻ��� Һ��pH |
| �� | 0.1 | 0.1 | pH=a |
| �� | 0.12 | 0.1 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=10 |
��2����������Һ��c��A-����c��Na+���Ĵ�С��ϵ��C��
A��ǰ�ߴ� B�����ߴ� C��������� D�����ж�
��3���ӱ���ʵ�����������û����Һ������Ũ���ɴ�С��˳����c��Na+����c��A-����c��OH-����c��H+����
��4����������ʵ�����ݣ�д���û����Һ��������ʽ�ľ�ȷ�������ʽ����
c��Na+��-c��A-��=10-4-10-10 mol•L-1��
��5��ij��Ԫ�ᣨ��ѧʽ��H2B��ʾ����ˮ�еĵ��뷽��ʽ�ǣ�H2B�TH++HB-
HB-?H++B2-��
��0.1mol•L-1��Na2B��Һ�У���������Ũ�ȹ�ϵʽ��ȷ����AC��
A��c��B2-��+c��HB-��=0.1mol•L-1
B��c��B2-��+c��HB-��+c��H2B��=0.1mol•L-1
C��c��OH-��=c ��H+��+c��HB-��
D��c��Na+��+c��OH-��=c��H+��+c��HB-��