��Ŀ����
��14�֣�J��L��M��R��T��ԭ��������������Ķ���������Ԫ�أ�J��R�����ڱ��е����λ�����±���JԪ��������ϼ۵ľ���ֵ����ԭ��������������ȣ�M�ǵؿ��к�������Ԫ�ء�

��1��Ԫ��T��������
��2��J����Ԫ����ɵĻ�����Aÿ��������4��ԭ����ɣ���֪���ȼ��a gA����ʱ����1mol������̼�����Һ̬ˮ�����ų�����b kJ����A����ȼ���ȵ��Ȼ�ѧ����ʽ��
��3����ҵȼ�ս�̿���β���к���M��R��ɵ����壬Ϊ�˲ⶨ�京��������ѡ����Լ���
��4�������ӹ�ҵ�У�L�������̬�⻯���ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ��
��5����L��M��ɵ� ���ּ�̬��ͬ�����廯��������ת������ͬ��ͬ�ݵļ��������зֱ����1mol��2mol��LM2���壬�ֱ���ƽ�⣬����������LM2��ת���ʱȽϼ� �ң�<��>��=��
���ּ�̬��ͬ�����廯��������ת������ͬ��ͬ�ݵļ��������зֱ����1mol��2mol��LM2���壬�ֱ���ƽ�⣬����������LM2��ת���ʱȽϼ� �ң�<��>��=��
��6����Jͬ�������һ������Ԫ�غ�M��ɵĻ������кܹ㷺����;����˵������ҪӦ�ã�д������

��1��Ԫ��T��������
��2��J����Ԫ����ɵĻ�����Aÿ��������4��ԭ����ɣ���֪���ȼ��a gA����ʱ����1mol������̼�����Һ̬ˮ�����ų�����b kJ����A����ȼ���ȵ��Ȼ�ѧ����ʽ��
��3����ҵȼ�ս�̿���β���к���M��R��ɵ����壬Ϊ�˲ⶨ�京��������ѡ����Լ���
| A������������Һ | B�������ữ�ĸ��������Һ | C�����۵⻯����Һ | D����ˮ��Һ |
��5����L��M��ɵ�
 ���ּ�̬��ͬ�����廯��������ת������ͬ��ͬ�ݵļ��������зֱ����1mol��2mol��LM2���壬�ֱ���ƽ�⣬����������LM2��ת���ʱȽϼ� �ң�<��>��=��
���ּ�̬��ͬ�����廯��������ת������ͬ��ͬ�ݵļ��������зֱ����1mol��2mol��LM2���壬�ֱ���ƽ�⣬����������LM2��ת���ʱȽϼ� �ң�<��>��=����6����Jͬ�������һ������Ԫ�غ�M��ɵĻ������кܹ㷺����;����˵������ҪӦ�ã�д������
��1���ȣ�2�֣�
��2����C2H2��g����5/2O2��g����2CO2��g����H2O��l���� ��H ����2b kJ / mol��3�֣�
��3��BD��2�֣�ѡ��һ��0�֣�ֻѡһ���ҶԸ�1�֣�
��4��2NH3��H2O + 3H2O2 =" " N2 + 8H2O ��3�֣�
��5������2�֣�
��6�� ���ά��ʯӢ������ʯӢ�ӱ����������ϵȣ�2�֣��𰸺������֣�
��2����C2H2��g����5/2O2��g����2CO2��g����H2O��l���� ��H ����2b kJ / mol��3�֣�
��3��BD��2�֣�ѡ��һ��0�֣�ֻѡһ���ҶԸ�1�֣�
��4��2NH3��H2O + 3H2O2 =" " N2 + 8H2O ��3�֣�
��5������2�֣�
��6�� ���ά��ʯӢ������ʯӢ�ӱ����������ϵȣ�2�֣��𰸺������֣�
��

��ϰ��ϵ�д�
 ��������ܸ�ϰϵ�д�
��������ܸ�ϰϵ�д�
�����Ŀ
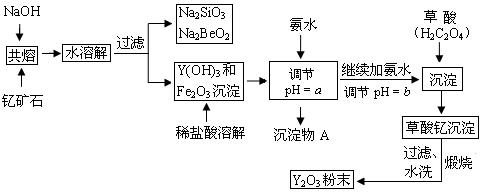
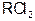 ����������Һʱ��Ϊ�˵õ�������Һ����ֹ���ʣ�������Һ�м�������R�ĵ��ʺ����ᡣ��֪
����������Һʱ��Ϊ�˵õ�������Һ����ֹ���ʣ�������Һ�м�������R�ĵ��ʺ����ᡣ��֪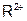 ��5�����Ӳ㣬���������2�����ӣ��������ƶ���ȷ����
��5�����Ӳ㣬���������2�����ӣ��������ƶ���ȷ����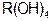 ��ǿ��
��ǿ��