��Ŀ����
���������У���ѧ�������ǣ� ��
| A��ϴ��������Ҫ�ɷ��DZ�����Լ������ܳ�ȥͷ���ϵ����� |
| B���÷�����Ӳˮϴͷ��ͷ����ɬ������������Ҫ����Ϊ�����еĸ�֬��������ˮ�е�Ca2+��Mg2+�γ��˲����Եĸ�֬����Ƶȸ�����ͷ���ϵ�Ե�� |
| C������Ⱦ���������������ɫ�����ɫ����ɫ����ɫ����ɫ����ɫ�ȣ�ʹ��ʱ�ɸ�ȡ���裬Ⱦ��һ������������ |
| D��Ⱦ�������ø���ʹͷ�������α�������Ĺ��̣������������� |
AB
A����ȷ��ϴ��������Ҫ�����ñ�����Լ���ȥͷ���ϵ����۵Ĺ��̡�����ϴ�������л��������������á�
B����ȷ�����ڸ�֬��������ˮ�е�Ca2+��Mg2+�γɵ��β�����ˮ���Ӷ�����ͷ����ɬ��
C�������ʱ��Ⱦ����һ����ˮ����Ⱦ��������ͷ�������Ⱦɫ������Ⱦ�����������������ɫ��������Ⱦ������������Ⱦ�������䱾����ɫ����������Գ���ɫ����һȾ�������ǻ�ѧ���̡�
D������̷�ʱ���������û�ԭ���ƻ�ͷ���еĻ�ѧ�����Ӷ�ʹͷ���������״̬������������������Σ�Ȼ���پ����������������ã���ͷ�����γ��µĻ�ѧ����ͷ���ָ������ԣ�ͬʱҲ�����˳־õIJ���״̬���ù���Ϊ��ѧ���̡�
B����ȷ�����ڸ�֬��������ˮ�е�Ca2+��Mg2+�γɵ��β�����ˮ���Ӷ�����ͷ����ɬ��
C�������ʱ��Ⱦ����һ����ˮ����Ⱦ��������ͷ�������Ⱦɫ������Ⱦ�����������������ɫ��������Ⱦ������������Ⱦ�������䱾����ɫ����������Գ���ɫ����һȾ�������ǻ�ѧ���̡�
D������̷�ʱ���������û�ԭ���ƻ�ͷ���еĻ�ѧ�����Ӷ�ʹͷ���������״̬������������������Σ�Ȼ���پ����������������ã���ͷ�����γ��µĻ�ѧ����ͷ���ָ������ԣ�ͬʱҲ�����˳־õIJ���״̬���ù���Ϊ��ѧ���̡�

��ϰ��ϵ�д�
�����Ŀ

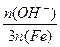 �� 100% ��ʽ��n(OH-)��n(Fe)�ֱ��ʾ����ۺ���������OH����FeԪ�ص����ʵ�������ش��������⣺
�� 100% ��ʽ��n(OH-)��n(Fe)�ֱ��ʾ����ۺ���������OH����FeԪ�ص����ʵ�������ش��������⣺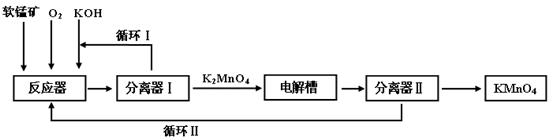
 2Fe+3CO2��CaCO3+SiO2
2Fe+3CO2��CaCO3+SiO2 RCOOR��+R��OH���˷�Ӧ��Ϊ��������Ӧ���������л��ϳ��С��ںϳ�ά�ڵĹ����У���һ�������ǰѾ�������ϩ��
RCOOR��+R��OH���˷�Ӧ��Ϊ��������Ӧ���������л��ϳ��С��ںϳ�ά�ڵĹ����У���һ�������ǰѾ�������ϩ��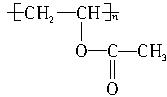 ת���ɾ���ϩ������һ�������ù����ļ״�������������Ӧ��ʵ�ֵġ�
ת���ɾ���ϩ������һ�������ù����ļ״�������������Ӧ��ʵ�ֵġ�