��Ŀ����
��2010?����һģ�����;�ˮ��������أ�K2FeO4��Ϊ����ɫ���壬������ˮ�������Ի�������Һ���ֽ⣬�ڼ�����Һ���ȶ�����ҵ���Ʊ�K2FeO4�ij��÷��������֣�
������������������������������ͼ��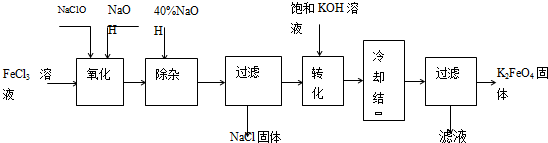
��1����ɡ������������з�Ӧ�Ļ�ѧ����ʽ��
��2����ת���������з�����Ӧ�Ļ�ѧ����ʽΪ
��3���������յõ��ĸ�����س��������ʣ������ؽᾧ���ᴿ�������ǣ����ֲ�Ʒ��
������ⷨ������Ϊ�����������������Һ��Ȼ��������Һ�м���KOH��
��4�����ʱ����������Ӧ����FeO42-���õ缫��Ӧ����ʽΪ
������������������������������ͼ��

��1����ɡ������������з�Ӧ�Ļ�ѧ����ʽ��
2
2
FeCl3+10
10
NaOH+3
3
NaClO��2
2
Na2FeO4+9NaCl
9NaCl
d+5H2O
5H2O
d������������NaClO
NaClO
���ѧʽ������2����ת���������з�����Ӧ�Ļ�ѧ����ʽΪ
Na2FeO4+2KOH=K2FeO4+2NaOH
Na2FeO4+2KOH=K2FeO4+2NaOH
����3���������յõ��ĸ�����س��������ʣ������ؽᾧ���ᴿ�������ǣ����ֲ�Ʒ��
ϡKOH��Һ
ϡKOH��Һ
�ܽ⣬Ȼ���ټ��뱥��KOH��Һ����ȴ�ᾧ������
�ټ��뱥��KOH��Һ����ȴ�ᾧ������
��������ⷨ������Ϊ�����������������Һ��Ȼ��������Һ�м���KOH��
��4�����ʱ����������Ӧ����FeO42-���õ缫��Ӧ����ʽΪ
Fe+8OH--6e-=FeO42-+4H2O
Fe+8OH--6e-=FeO42-+4H2O
����������1����Ӧ��NaClO������������ԭ������NaCl������Ԫ���غ㣬��֪��Ӧʽ����Ҫ����NaCl��H2O�����ݻ��ϼ���������ƽ����ʽ��
��2���ɹ������̿�֪�������������̳��Ӻ����Һ�к���Na2FeO4����ת�������̵IJ���ΪK2FeO4���ʡ�ת�����������ڼ���KOH��Һ��Na2FeO4ת��Ϊ�ܽ�ȸ�С��K2FeO4��
��3������Ŀ��Ϣ��֪��K2FeO4������ˮ�������Ի�������Һ���ֽܷ⣬�ڼ�����Һ���ȶ����ڷ������ᴿ��ʱ���Ҫ�ڼ��Ի����н��У�Ҫ��ֹ���������ʣ�������Ҫ��K2FeO4�ֲ�Ʒ��ϡKOH��Һ���ܽ⣬Ȼ����뱥��KOH��Һ����ȴ�ᾧ��
��4������Ŀ��Ϣ��֪����Ϊ�����������������Һ������FeO42-����Ԫ���غ㻹����ˮ��
��2���ɹ������̿�֪�������������̳��Ӻ����Һ�к���Na2FeO4����ת�������̵IJ���ΪK2FeO4���ʡ�ת�����������ڼ���KOH��Һ��Na2FeO4ת��Ϊ�ܽ�ȸ�С��K2FeO4��
��3������Ŀ��Ϣ��֪��K2FeO4������ˮ�������Ի�������Һ���ֽܷ⣬�ڼ�����Һ���ȶ����ڷ������ᴿ��ʱ���Ҫ�ڼ��Ի����н��У�Ҫ��ֹ���������ʣ�������Ҫ��K2FeO4�ֲ�Ʒ��ϡKOH��Һ���ܽ⣬Ȼ����뱥��KOH��Һ����ȴ�ᾧ��
��4������Ŀ��Ϣ��֪����Ϊ�����������������Һ������FeO42-����Ԫ���غ㻹����ˮ��
����⣺��1����Ӧ��FeCl3��Na2FeO4����Ԫ�ػ��ϼ���+3������Ϊ+6�ۣ����ϼ�������3�ۣ�NaClO��NaCl����Ԫ�ػ��ϼ���+1����Ϊ-1�ۣ����ϼ��ܹ�����2�ۣ����ϼ�������С������Ϊ6����FeCl3ϵ��Ϊ2��NaClOϵ��Ϊ3������Ԫ���غ��֪ Na2FeO4ϵ��Ϊ2������Ԫ���غ��֪NaClϵ��Ϊ2��3+3=9��������Ԫ���غ��֪NaOHϵ��Ϊ9+2��2=13������Ԫ���غ��֪H2Oϵ��Ϊ5����ƽ����ʽΪ2FeCl3+10NaOH+3NaClO=2Na2FeO4+9NaCl+5H2O��
��Ӧ��NaClO��NaCl����Ԫ�ػ��ϼ���+1����Ϊ-1�ۣ�NaClO������������ԭ������NaCl��
�ʴ�Ϊ��2��10��3��2��9NaCl��5H2O��NaClO��
��2����ת�����������ڼ���KOH��Һ��Na2FeO4ת��Ϊ�ܽ�ȸ�С��K2FeO4����Ӧ����ʽΪNa2FeO4+2KOH=K2FeO4+2NaOH��
�ʴ�Ϊ��Na2FeO4+2KOH=K2FeO4+2NaOH��
��3������Ŀ��Ϣ��֪��K2FeO4������ˮ�������Ի�������Һ���ֽܷ⣬�ڼ�����Һ���ȶ����ڷ������ᴿ��ʱ���Ҫ�ڼ��Ի����н��У�Ҫ��ֹ���������ʣ�������Ҫ��K2FeO4�ֲ�Ʒ��ϡKOH��Һ���ܽ⣬Ȼ����뱥��KOH��Һ����ȴ�ᾧ�����ˣ�
�ʴ�Ϊ��ϡKOH��Һ���ټ��뱥��KOH��Һ����ȴ�ᾧ�����ˣ�
��4������Ŀ��Ϣ��֪����Ϊ�����������������Һ������FeO42-�������缫��ӦʽΪFe+8OH--6e-=FeO42-+4H2O��
�ʴ�Ϊ��Fe+8OH--6e-=FeO42-+4H2O��
��Ӧ��NaClO��NaCl����Ԫ�ػ��ϼ���+1����Ϊ-1�ۣ�NaClO������������ԭ������NaCl��
�ʴ�Ϊ��2��10��3��2��9NaCl��5H2O��NaClO��
��2����ת�����������ڼ���KOH��Һ��Na2FeO4ת��Ϊ�ܽ�ȸ�С��K2FeO4����Ӧ����ʽΪNa2FeO4+2KOH=K2FeO4+2NaOH��
�ʴ�Ϊ��Na2FeO4+2KOH=K2FeO4+2NaOH��
��3������Ŀ��Ϣ��֪��K2FeO4������ˮ�������Ի�������Һ���ֽܷ⣬�ڼ�����Һ���ȶ����ڷ������ᴿ��ʱ���Ҫ�ڼ��Ի����н��У�Ҫ��ֹ���������ʣ�������Ҫ��K2FeO4�ֲ�Ʒ��ϡKOH��Һ���ܽ⣬Ȼ����뱥��KOH��Һ����ȴ�ᾧ�����ˣ�
�ʴ�Ϊ��ϡKOH��Һ���ټ��뱥��KOH��Һ����ȴ�ᾧ�����ˣ�
��4������Ŀ��Ϣ��֪����Ϊ�����������������Һ������FeO42-�������缫��ӦʽΪFe+8OH--6e-=FeO42-+4H2O��
�ʴ�Ϊ��Fe+8OH--6e-=FeO42-+4H2O��
���������鷽��ʽ����д��������ʵ��������Ķ���Ŀ��ȡ��Ϣ�����ȣ��Ѷ��еȣ���Ҫѧ���߱��ۺ�����֪ʶ����Ŀ��Ϣ�������⡢�����������������������Ŀ����Ҫ�����ÿһ����Ӧ�����������������ԭ����

��ϰ��ϵ�д�
 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
�����Ŀ