��Ŀ����
18��I���������Ժʿ����Ժʿѧ��ͬ���ġ����ӹ��������ն�����������ˣ�ȫ������������Ϸ˵��BF3��TiO2��CH3COOH��CO2��NO����ï����NH3��HCN��H2S��O3�������ϩ���Ƶ��ڶࡰ���ӹ������е����ǣ�
��1��д��Fe2+�ĺ�������Ų�ʽ[Ar]3d6��
��2������˵����ȷ����cd��
a��H2S��O3���Ӷ���ֱ����
b��BF3��NH3���Ǻ��м��Լ��ķǼ��Է���
c��CO2��HCN���ӵĽṹʽ�ֱ��ǣ�O=C=O��H-C��N
d��CH3COOH������̼ԭ�ӵ��ӻ���ʽ�У�sp2��sp3
��3��NO�������е��źŷ��ӣ��й�˵����ȷ��abcd��
a��ԭ�Ӱ뾶N����O b���ǽ�����O����N
c��NO+�ĽṹʽΪ����N��O��+�� d��NO�γɵľ����Ƿ��Ӿ���
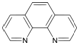
��4��TiO2����Ȼ�����У����ȶ���һ�־���ṹ��ͼ1�������ʾOԭ�ӣ�
��5�������۷е�ܸߣ������ڴ����Է��Ӽ�����ϵĶ����壨��һ����״�ṹ�����뻭���������Ľṹ��
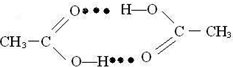 ��
����6����ï����C5H5��2Fe��Fe2+�뻷���ϩ���γɵ�һ�����
�ʵ���Ҳⶨ���ĺ�����������λ���ڶ�����
��
 ����������Fe2+�γɺ�ɫ��������ͼ2����
����������Fe2+�γɺ�ɫ��������ͼ2��������������Fe2+�뵪ԭ���γ���λ������6����
���� ��1������26��Ԫ�أ���ԭ�Ӻ�����26�����ӣ���ԭ��ʧȥ2�����ӱ�ΪFe2+�����ݹ���ԭ��д��Fe2+��̬���Ӻ�������Ų�ʽ��
��2�����ݼ۲���ӶԻ�������ȷ�����Ŀռ乹�ͣ���ͬ�ǽ���Ԫ��֮���γɼ��Լ���ͬ�ַǽ���Ԫ��֮���γɷǼ��Թ��ۼ���
��3��a��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ���������������С��
b��ͬһ����Ԫ�أ�Ԫ�صķǽ���������ԭ���������������ǿ��
c���������Ӻ��ǵȵ����壬�ȵ�����ṹ���ƣ��Ե������ӵĽṹ��дNO+�Ľṹ��
d�����Ӿ���Ĺ������Ƿ��ӣ�
��4�����þ�̯���������ԭ�Ӹ������ٽ�ϻ�ѧʽ�жϣ�
��5���ڶ���������Ӽ��У��Ȼ��ϵ���ԭ������һ�����������̼��˫������ԭ���γ������
��6�����йµ��ӶԵ�ԭ�Ӻͺ��пչ�������Ӽ����γ���λ����
��� �⣺��1������26��Ԫ�أ���ԭ�Ӻ�����26�����ӣ���ԭ��ʧȥ2�����ӱ�ΪFe2+�����ݹ���ԭ��֪�������Ӻ�������Ų�ʽΪ[Ar]3d6��
�ʴ�Ϊ��[Ar]3d6��
��2��a��H2S��O3���Ӷ���V�Σ��ʴ���
b��BF3��NH3���Ǻ��м��Լ�����ǰ���ǷǼ��Է��ӣ��������Ǽ��Է��ӣ��ʴ���
c��CO2��HCN���ӵĽṹʽ�ֱ��ǣ�O=C=O��H-C��N������ȷ��
d��CH3COOH������̼ԭ�ӵļ۲���Ӷ����ֱ�Ϊ4��3�������ӻ���ʽ�У�sp3��sp2������ȷ��
��ѡcd��
��3��a��ͬһ����Ԫ�أ�ԭ�Ӱ뾶����ԭ���������������С������ԭ�Ӱ뾶N����O������ȷ��
b��ͬһ����Ԫ�أ�Ԫ�صķǽ���������ԭ���������������ǿ�����Էǽ�����O����N������ȷ��
c���������Ӻ��ǵȵ����壬�ȵ�����ṹ���ƣ��Ե������ӵĽṹ֪NO+�ĽṹΪN��O+������ȷ��
d�����Ӿ���Ĺ������Ƿ��ӣ�NO�γɵľ��幹������NO���ӣ�����NO�������ڷ��Ӿ��壬����ȷ��
��ѡabcd��
��4���������=2+4��$\frac{1}{2}$=4���������=1+8��$\frac{1}{8}$��������ͺ��������Ϊ2��1�����ݶ�������Ļ�ѧʽ֪�������ʾ��ԭ�ӣ�
�ʴ�Ϊ��O��
��5������������Ӽ��У��Ȼ��ϵ���ԭ������һ�����������̼��˫������ԭ���γ�����������������ʾΪ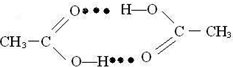 ��
��
�ʴ�Ϊ��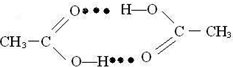 ��
��
��6����������������������ӣ��ṩ�չ������ԭ���ṩ�µ��Ӷԣ�����ͼ֪����λ��������6��
�ʴ�Ϊ��6��
���� ���⿼����ۺϣ��漰��������Ӽ��Ե��жϡ���������Ų�ʽ����д��֪ʶ�㣬�ѵ����������д����ȷ��ЩԪ�ص�ԭ�����γ�������Ѷ��еȣ�

 ���ס���Ϊ�����ڡ�ͬһ����Ԫ����ɵĵ��ʣ��ҡ�������������Ԫ����ɵĻ��������֮������ͼ��ʾ��ת����ϵ�������������ļͱ�����Ϊ��������
���ס���Ϊ�����ڡ�ͬһ����Ԫ����ɵĵ��ʣ��ҡ�������������Ԫ����ɵĻ��������֮������ͼ��ʾ��ת����ϵ�������������ļͱ�����Ϊ��������| A�� | �ƺ����� | B�� | ������� | C�� | ̼�� | D�� | �������嵥�� |
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
| A�� | ����33.6LN2����״���£� | B�� | ����ԭ��Nԭ�ӵ����ʵ���Ϊ2mol | ||
| C�� | ת�Ƶ��ӵ����ʵ���Ϊ4 mol | D�� | ��ԭ��������������0.5mol |
| A�� | 6.0gSiO2�������0.2NA��Si-O�� | |
| B�� | 1L1mol•L-1CH3COOH��Һ�У�����CH3COO-��CH3COOH������ΪNA | |
| C�� | 1L1mol•L-1����FeCl3��Һ�����ˮ����ȫˮ������Fe��OH��3����������ΪNA�� | |
| D�� | 10g46%���Ҵ�ˮ��Һ������Hԭ����Ϊ0.6NA |