��Ŀ����
5������������ԭ��ת�������÷dz���Ҫ�����з�Ӧ��CCl4+H2?CHCl3+HCl���������Ͽ�֪�е㣺CCl4Ϊ77�棬CHCl3Ϊ61.2�森���ܱ������У��÷�Ӧ�ﵽƽ�����±����ݣ����費���Ǹ���Ӧ����| ʵ�� ��� | �¶ȡ� | ��ʼCCl4Ũ�� ��mol•L-1�� | ��ʼH2Ũ�ȣ�mol•L-1�� | CCl4��ƽ��ת���� |
| 1 | 110 | 1 | 1.2 | a |
| 2 | 110 | 1 | 1 | 50% |
| 3 | 100 | 1 | 1 | b |
��2����ʵ��2�У���1Сʱ��Ӧ�ﵽƽ�⣬��H2��ƽ����Ӧ����Ϊ0.5mol/��L��h�����ڴ�ƽ����ϵ���ټ���0.5molCCl4��1.0molHCl��ƽ�⽫���� �����ƶ���������������ƶ���
��3��ʵ��1�У�CCl4��ת����a��50%�����������=����������ʵ��3�У�b��ֵD ������ĸ��ţ���
A������50% B������50% C��С��50% D���ɱ��������������жϣ�
���� ��1����ѧƽ�ⳣ����ָ��һ���¶��£����淴Ӧ����ƽ��ʱ���������Ũ��ϵ������֮���뷴Ӧ���Ũ��ϵ������֮���ıȣ����塢��Һ�岻��Ҫ�ڻ�ѧƽ�ⳣ����д����
110��ʱ��ʵ��2�дﵽƽ��ʱCCl4��ת����Ϊ50%��ƽ��ʱCCl4��Ũ�ȱ仯��=1mol/L��50%=0.5mol/L����
CCl4��g��+H2��g��?CHCl3��g��+HCl��g��
��ʼ��mol/L����1 1 0 0
�仯��mol/L����0.5 0.5 0.5 0.5
ƽ�⣨mol/L����0.5 0.5 0.5 0.5
����ƽ�ⳣ��K=$\frac{c��CHC{l}_{3}����c��HCl��}{c��CC{l}_{4}����c��{H}_{2}��}$���㣻
��2������v=$\frac{��c}{��t}$����������ʾ�ķ�Ӧ���ʣ�
�ж�Ũ����Qc��ƽ�ⳣ����Դ�С����Qc=K������ƽ��״̬����Qc��K����Ӧ������Ӧ���У���Qc��K����Ӧ���淴Ӧ���У�
��3��ʵ��1��2�¶���ͬ��ʵ��1��Ч��ʵ��2�Ļ�������������Ũ�ȣ�ƽ�������ƶ���ʵ��1�е�CCl4��ת���ʽϴ�
����ʵ��3�����¶Ȳ�ͬ���ֲ�֪�÷�Ӧ����ЧӦ���������ж�ת���ʵĴ�С��
��� �⣺��1����CCl4�ķе�Ϊ77�棬CHCl3�ķе�Ϊ61.2�棬������100�淴Ӧʱ�����ʾ�Ϊ��̬��CCl4��g��+H2��g��?CHCl3��g��+HCl��g����ƽ�ⳣ������ʽK=$\frac{c��CHC{l}_{3}����c��HCl��}{c��CC{l}_{4}����c��{H}_{2}��}$��
110��ʱ��ʵ��2�дﵽƽ��ʱCCl4��ת����Ϊ50%��ƽ��ʱCCl4��Ũ�ȱ仯��=1mol/L��50%=0.5mol/L����
CCl4��g��+H2��g��?CHCl3��g��+HCl��g��
��ʼ��mol/L����1 1 0 0
�仯��mol/L����0.5 0.5 0.5 0.5
ƽ�⣨mol/L����0.5 0.5 0.5 0.5
��ƽ�ⳣ��K=$\frac{c��CHC{l}_{3}����c��HCl��}{c��CC{l}_{4}����c��{H}_{2}��}$=$\frac{0.5��0.5}{0.5��0.5}$=1��
�ʴ�Ϊ��$\frac{c��CHC{l}_{3}����c��HCl��}{c��CC{l}_{4}����c��{H}_{2}��}$��1��
��2��ʵ��2�У���1Сʱ��Ӧ�ﵽƽ�⣬��H2��ƽ����Ӧ����Ϊ$\frac{0.5mol/L}{1h}$=0.5mol/��L��h����
�ڴ�ƽ����ϵ���ټ���0.5molCCl4��1.0molHCl����K=$\frac{c��CHC{l}_{3}����c��HCl��}{c��CC{l}_{4}����c��{H}_{2}��}$��֪��Ũ����Qc��K=1����Ӧ���淴Ӧ���У�
�ʴ�Ϊ��0.5mol/��L��h�����棻
��3��ʵ��1��2�¶���ͬ��ʵ��1��Ч��ʵ��2�Ļ�������������Ũ�ȣ�ƽ�������ƶ���ʵ��1�е�CCl4��ת���ʽϴ�CCl4��ת����a��50%��
����ʵ��3�����¶���ʵ��1��2��ͬ���ֲ�֪�÷�Ӧ����ЧӦ���������ж�ת���ʵĴ�С��
�ʴ�Ϊ������D��
���� ���⿼�黯ѧƽ�������Ӱ�����ء���ѧƽ�ⳣ�����㼰Ӧ�õȣ��Ѷ��еȣ�ע����������ʽ������ƽ�ⳣ����Ӧ�ã�

 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�| A�� | ��AgNO3��Һ�еμ����Ag++Cl-=AgCl�� | |
| B�� | ��Ba��OH��2��Һ�м�������Һ��Ba2++SO42-=BaSO4�� | |
| C�� | ���ô��ᣨCH3COOH���ܽ⺬̼��Ƶ�ˮ����CaCO3+2H+=Ca2++H2O+CO2�� | |
| D�� | ������Һ�еμ�NaHCO3��Һ��2H++CO32-=CO2��+H2O |
| A�� | ��ʳͭ��������ţ�̺͵��� | |
| B�� | þ���ͽ������Ż�ʱ��ɳ����� | |
| C�� | Ƥ����մ�б��ӣ�Ӧ�����ô���ϡNaOH��ϴ | |
| D�� | ��Һ����ľ���ϣ������������������ϡ�����кͣ�Ȼ����ˮ��ϴ |
| A�� | ��ԭ������һ�������ܱ���ȶ��ṹ����Ҳ�ɽ������ڵڢ�A�� | |
| B�� | ��Ԫ��������Ԫ���γɻ�����ʱ�����ϼ�ֻ��+1�� | |
| C�� | ��������������һ���������ܶȱȿ���С���ŷɺ����ڿն��� | |
| D�� | ���þ�����ڱ���λ�ڶԽ���λ�ã����Ƶ�Li0HΪ��ǿ�� |
| A�� | c��NH4+��=c��Na+��=c��OH-����c��NH3•H2O�� | B�� | c��NH4+��=c��Na+����c��NH3•H2O����c��OH-����c��H+�� | ||
| C�� | c��NH4+����c��Na+����c��OH-����c��NH3•H2O�� | D�� | c��NH4+����c��Na+����c��NH3•H2O����c��OH-����c��H+�� |
| A�� | ��ȥCO2�л��е�HCl���ɽ�����ͨ������̼������Һ | |
| B�� | п��Ͷ��ϡ�����У����������ӷ�Ӧ�ǣ�Zn+2H+�TZn2++H2�� | |
| C�� | ��ȥ���������������ӣ������������ķ��� | |
| D�� | �����£���Ũ�ȵ�����Ϳ�����Һ��ǰ�ߵĵ����Ը�ǿ |
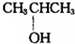 ��$\stackrel{13}{6}$C ��CH3OH��
��$\stackrel{13}{6}$C ��CH3OH��