��Ŀ����
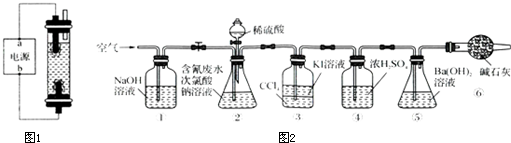
��1��ʵ��I ��ȡ����������Һ����ʯī���缫��ⱥ���Ȼ�����Һ��ȡ����������Һ�����ɴ������Ƶ����ӷ���ʽΪ
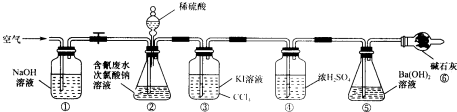
ʵ���ⶨ�����ˮ�����ٷ��ʣ�������ͼ��ʾװ�ý���ʵ�飺��CN-��Ũ��Ϊ0.2000mol?L-1�ĺ����ˮ100mL��l00mL NaClO��Һ������������װ�â���ƿ�г�ַ�Ӧ����Һ©������������100ml��ϡH2SO4���رջ�����
��֪װ�â��з�������Ҫ��Ӧ����Ϊ��
CN-+ClO-�TCNO+Cl-
2CNO-+2H++3ClO-�TN2��+2CO2��+3Cl-+H2O
��2��װ�â۵�������
��3����Ӧ��������ͨ�������Ŀ����
��4��Ϊ�����ʵ���к����ˮ�������İٷ��ʣ���Ҫ�ⶨ
��5������ʵ����ɺ�Ϊ�˻���װ�â��е�CCl4��Ҫ�IJ�����
��6����Ҫ�о�װ�â�������ϡ��������Ũ�ȣ�����д��װ�ý���ʵ��ļ�¼����Ҫ���ڽ��������жϺ�����Ũ����Ϊ��һ��ʵ�����ݣ��ڼ�¼��Ҫ����ʵ������еIJ��������Ա������������
| ʵ�� | c��H2SO4��mol��L-1 | V��H2SO4��mL | V��NaClO��mL | ���ȷ�ˮ�����mL |
| 1 | 100 | 100 | ||
| 2 | 0.0500 | 100 | 100 | |
| 3 | 0.2000 | 100 | 100 |
��2��ʵ���ԭ��������CN-+ClO-�TCNO+Cl-��2CNO-+2H++3ClO-�TN2��+2CO2��+3Cl-+H2O��ͨ���ⶨ��ʯ�ҵ������ı仯��ö�����̼�����������ݹ�ϵʽ���㺬���ˮ�����ٷ��ʣ�ʵ����Ӧ�ų������Ϳ����ж�����̼�ĸ��ţ�
��3����Ӧ��������ͨ��������������ɵĶ�����̼ȫ�������գ�
��4��ͨ���ⶨ��ʯ�ҵ������ı仯��ö�����̼�����������ݹ�ϵʽ���㺬���ˮ�����ٷ��ʣ�
��5������װ�â��е�CCl4��Ҫ��Һ�����������
��6���о�װ�â�������ϡ��������Ũ�ȣ�Ӧ�����������NaClO�����ͬ���������ò�ͬŨ�ȵ�������ж���ʵ�飮
��Ӧ��������ӷ���ʽΪCl-+H2O
| ||
�ʴ�Ϊ��Cl-+H2O
| ||
��2��ʵ���ԭ��������CN-+ClO-�TCNO+Cl-��2CNO-+2H++3ClO-�TN2��+2CO2��+3Cl-+H2O��ͨ���ⶨ��ʯ�ҵ������ı仯��ö�����̼�����������ݹ�ϵʽ���㺬���ˮ�����ٷ��ʣ�ʵ����Ӧ�ų������Ϳ����ж�����̼�ĸ��ţ���װ�â۵�����������װ�â��п��ܲ�����Cl2����ֹ��װ�â�ʵ�����ݵIJⶨ�������ţ�װ�â�������
�ų������ж�����̼��ʵ��ĸ��ţ�
�ʴ�Ϊ������װ�â��п��ܲ�����Cl2����ֹ��װ�â�ʵ�����ݵIJⶨ�������ţ��ų������ж�����̼��ʵ��ĸ��ţ�
��3����Ӧ��װ���в���������̼��Ӧ����ͨ���������Ŀ�������װ���ڵIJ����Ķ�����̼ȫ������װ�âݣ��Լ���ʵ����
�ʴ�Ϊ��ʹװ���в����Ķ�����̼ȫ������װ�âݣ�
��4��ͨ���ⶨ��ʯ�ҵ������ı仯��ö�����̼�����������ݹ�ϵʽ���㺬���ˮ�����ٷ��ʣ�����Ҫ�ⶨװ�âݷ�Ӧǰ���������
�ʴ�Ϊ��װ�âݷ�Ӧǰ��
��5�����Ȼ�̼������ˮ����ͨ����Һ���룬Ȼ������ɵõ���
�ʴ�Ϊ����Һ������
��6���о�װ�â�������ϡ��������Ũ�ȣ�Ӧ�����������NaClO�����ͬ���������ò�ͬŨ�ȵ�������ж���ʵ�飬��1�������Ũ�ȿ�Ϊ0.100mol/L��
�ʴ�Ϊ��
| ʵ�� | c��H2SO4��mol��L-1 | V��H2S O4��mL | V��NaClO��mL | ���ȷ�ˮ�����mL |
| 1 | 0.100 | 100 | ||
| 2 | 100 | |||
| 3 | 100 |

��1����ˮ������������Եõ���ˮ�Ȼ�þ����ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϣ���д������ˮ�Ȼ�þ��ȡ����þ�Ļ�ѧ��Ӧ����ʽ
��2��ij���������������л��������Cu2+��Pb2+����ˮ���ŷ�ǰ���ó�������ȥ���������ӣ������������ݣ�����ΪͶ��
| ���ܵ���� | Cu��OH��2 | CuS | Pb��OH��2 | PbS | Ksp | 4.8��10-20 | 6.3��10-36 | 1.2��10-15 | 1.0��10-28 |
����Ȼ��ˮ��pH��8���������ӷ���ʽ������Ȼ��ˮ�������Ե�ԭ��
��ij�о�С��Ϊ̽����ߺ���������SO2������Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ������ͼ��
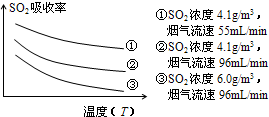
�������ͼʾʵ��������������һ��Ũ�Ⱥ���������SO2������Ч�ʣ����һ�����������飺
����Ȼ��ˮ�����˺��������������H2SO3��HSO3-�ȷ��ӻ����ӣ�ʹ���������������Ļ�ѧԭ����
�����غ��������ŷḻ�ĺ�ˮ��Դ����ˮ����Ҫ����Na����K����Ca2����Mg2����Cl����SO42����Br����CO32����HCO3�������ӡ�����������Դ�ͱ��������ǿɳ�����չ����Ҫ��֤��
 ��1����ˮ������������Եõ���ˮ�Ȼ�þ����ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϡ���д����������Ȼ�þ��ȡ����þ�Ļ�ѧ��Ӧ����ʽ???????????????????????????????? ��
��1����ˮ������������Եõ���ˮ�Ȼ�þ����ˮ�Ȼ�þ�ǹ�ҵ��ȡþ��ԭ�ϡ���д����������Ȼ�þ��ȡ����þ�Ļ�ѧ��Ӧ����ʽ???????????????????????????????? ��
��2��ij���������������л��������Cu2����Pb2������ˮ���ŷ�ǰ���ó�������ȥ���������ӣ������������ݣ�����ΪͶ��?????????? ��ѡ����Na2S������NaOH����Ч�����á�
���ܵ���� | Cu(OH)2 | CuS | Pb(OH)2 | PbS |
Ksp | 4��8��10��20 | 6��3��10��36 | 1��2��10��15 | 1��0��10��28 |
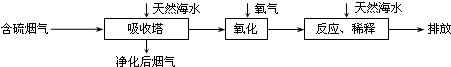
��3�������������ҹ�����Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⡣���ú�ˮ������һ����Ч�ķ������乤����������ͼ��ʾ��

����Ȼ��ˮ��pH��8���������ӷ���ʽ������Ȼ��ˮ�������Ե�ԭ��????????? ����дһ������
��ij�о�С��Ϊ̽����ߺ���������SO2����Ч�ʵĴ�ʩ����������Ȼ��ˮ���պ���������ģ��ʵ�飬ʵ������ͼ��ʾ��

�������ͼʾʵ��������������һ��Ũ�Ⱥ���������SO2������Ч�ʣ����һ�����������飺??? ��
����Ȼ��ˮ�����˺��������������H2SO3��HSO3���ȷ��ӻ����ӣ�ʹ���������������Ļ�ѧԭ����?????????????????? ����дһ����ѧ����ʽ�����ӷ���ʽ���������������ˮ����Ҫ�����������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ����???????????????????????? ��
������Դ����������ͷ�չ����Ҫ֧�����о���ѧ��Ӧ�����е������仯����Դ��ȱ�Ľ��������Ҫ���������塣��֪�����Ȼ�ѧ����ʽ
��2H2(g)+O2(g)��2H2O(l)????  H����570kJ/mol��
H����570kJ/mol��
��H2(g)+1/2O2(g)��H2O(g)???  H����242kJ/mol��
H����242kJ/mol��
��C(s)+1/2O2(g)��CO(g)????  H����110��5kJ/moL��
H����110��5kJ/moL��
��C(s)+O2(g)��CO2(g)???????  H����393��5kJ/moL��
H����393��5kJ/moL��
��CO2(g)+2H2O(g)��2CH4(g)+2 O2(g)?  H��+890kJ/moL
H��+890kJ/moL
�ش���������
��1��������Ӧ���������ȷ�Ӧ����??????????????? ��
��2��H2��ȼ����Ϊ��H��??????????????? ��
��3����˹�����������Ϳ�ѧ�о����к���Ҫ�����塣��Щ��Ӧ�ķ�Ӧ����Ȼ��ֱ�Ӳⶨ������ͨ����ӵķ�����á���֪C(s) + H2O(g)��H2(g)+ CO(g)????  H��akJ/moL����a��???????? ���÷�Ӧ����
H��akJ/moL����a��???????? ���÷�Ӧ���� S???????? 0(ѡ������������������������)����֪������
S???????? 0(ѡ������������������������)����֪������ G��
G�� H��T
H��T S����
S���� G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է�����__________________��
G��0ʱ���Է����С���÷�Ӧ��ʲô�����¿��Է�����__________________��
��4��CO��������ȼ�ϵ��Ϊ����ԭ������װ����ͼ��ʾ���õ���е����Ϊ�����ƣ������ƣ�����O2-�����ڹ������NASICON�������ƶ�������˵���������????? ��

A�������ĵ缫��ӦʽΪ��CO+O2���D2e-��CO2
B������ʱ�缫b��������O2�����缫a����缫b
C������ʱ�����ɵ缫aͨ������������缫b
D����������ͨ��������Խ����β����CO�ĺ���Խ��
ij�о�С��ģ�ҵ��Ĥ��ⷨ������ƺ����ˮ�����������й�ʵ�飬�ش��������⣮
��1��ʵ��I����ȡ����������Һ����ʯī���缫��ⱥ���Ȼ�����Һ��ȡ����������Һ�����ɴ������Ƶ����ӷ���ʽΪ______��
ʵ���ⶨ�����ˮ�����ٷ��ʣ�������ͼ��ʾװ�ý���ʵ�飺��CN-��Ũ��Ϊ0.2000mol?L-1�ĺ����ˮ100mL��l00mL NaClO��Һ������������װ�â���ƿ�г�ַ�Ӧ����Һ©������������100ml��ϡH2SO4���رջ�����

��֪װ�â��з�������Ҫ��Ӧ����Ϊ��
CN-+ClO-�TCNO+Cl-
2CNO-+2H++3ClO-�TN2��+2CO2��+3Cl-+H2O
��2��װ�â۵�������______��װ�â�������______��
��3����Ӧ��������ͨ�˿�����Ŀ����______��
��4��Ϊ�����ʵ���к����ˮ�������İٷ��ʣ���Ҫ�ⶨ______��������
��5������ʵ����ɺ�Ϊ�˻���װ�â��е�CCl4��Ҫ�IJ�����______��
��6����Ҫ�о�װ�â�������ϡ��������Ũ�ȣ�����д��װ�ý���ʵ��ļ�¼����Ҫ���ڽ��������жϺ�����Ũ����Ϊ��һ��ʵ�����ݣ��ڼ�¼��Ҫ����ʵ������еIJ��������Ա������������
| ʵ�� | c��H2SO4��mol��L-1 | V��H2S O4��mL | V��NaClO��mL | ���ȷ�ˮ�����mL |
| 1 | ______ | 100 | ______ | 100 |
| 2 | 0.0500 | ______ | 100 | 100 |
| 3 | 0.2000 | ______ | 100 | 100 |