��Ŀ����
A�����ĺ��������Ϊ�˲ⶨ�������ɣ���������ʵ�飺
����һ���¶Ⱥ�ѹǿ�½�A�������������������ͬ�¡�ͬѹ�µ��������������38�����ڳ�ȡ7.6gA����11.2L��������ȫȼ�գ������ֻ��CO2��ˮ����������Ӧ��Ļ����ͨ��Ũ����������ܵ����ʵ���Ϊ0.475mol��ŨH2SO4��������3.6g���ٽ����µ�����ͨ��ʢ������Na2O2�ĸ���ܺ���������ʵ���������0.275mol(����������ڱ�״���²ⶨ)��
�Իش�
��1��A�Ļ�ѧʽΪ_____________��
��2��A������ֻ��һ��֧������FeCl3��Һ����ɫ��Ӧ��1molAֻ����1molNaOH��Ӧ��1molA������Na��Ӧ����1molH2����A�Ľṹ��ʽΪ__________________��
����һ���¶Ⱥ�ѹǿ�½�A�������������������ͬ�¡�ͬѹ�µ��������������38�����ڳ�ȡ7.6gA����11.2L��������ȫȼ�գ������ֻ��CO2��ˮ����������Ӧ��Ļ����ͨ��Ũ����������ܵ����ʵ���Ϊ0.475mol��ŨH2SO4��������3.6g���ٽ����µ�����ͨ��ʢ������Na2O2�ĸ���ܺ���������ʵ���������0.275mol(����������ڱ�״���²ⶨ)��
�Իش�
��1��A�Ļ�ѧʽΪ_____________��
��2��A������ֻ��һ��֧������FeCl3��Һ����ɫ��Ӧ��1molAֻ����1molNaOH��Ӧ��1molA������Na��Ӧ����1molH2����A�Ľṹ��ʽΪ__________________��
��1��C8H8O3 ��2��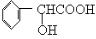
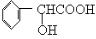
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

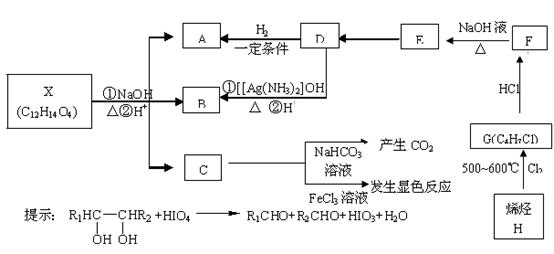
 G���ߣߣߣߣߣߣߣߣߣߣߣߣ���Ӧ���ͣ��ߣߣߣߣߣ�
G���ߣߣߣߣߣߣߣߣߣߣߣߣ���Ӧ���ͣ��ߣߣߣߣߣ�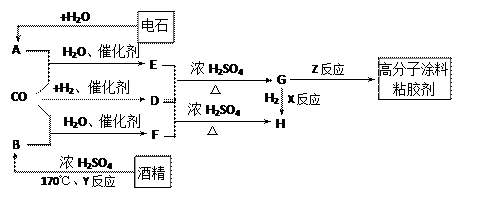
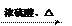
 ��4��д������ת���Ļ�ѧ����ʽ��
��4��д������ת���Ļ�ѧ����ʽ��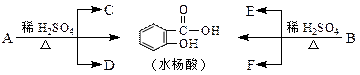
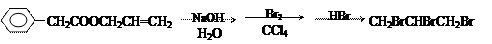
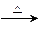
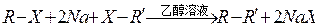

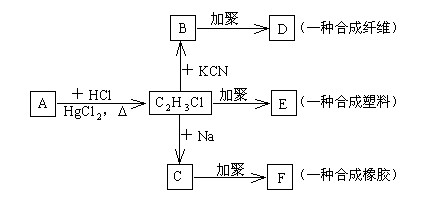


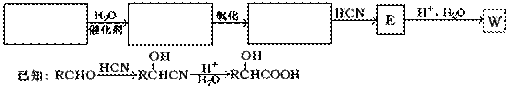
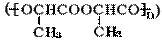 ���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ�� ��
���۱���������˿���Ƴ���������ߣ��������ڿ��Զ���������ΪW���۱����������ڽ���Ļ�ѧ����ʽ�� ��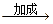
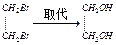
 CH2==CH2
CH2==CH2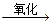 CH3CHO
CH3CHO