��Ŀ����
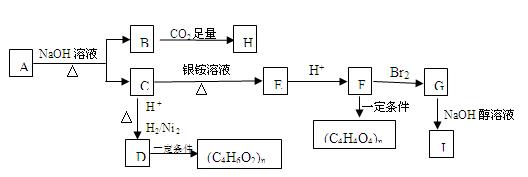
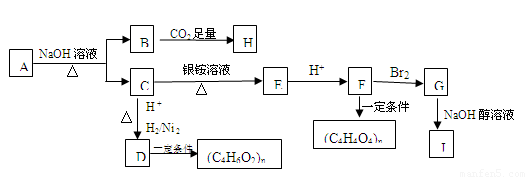
ij���ĺ���������A�ܷ�������ͼ��ʾ�ı仯����֪A�������ܶ�����ͬ������H2��88����C������̼ԭ����ͬһƽ���ϣ�J����������̼ԭ����һ��ֱ���ϣ�H��FeCl3��Һ����ɫ��
��1��A�����к��еĹ������� ��
A��B+C�ķ�Ӧ����ʽΪ ��
��2��д����B����H�ķ�Ӧ����ʽΪ ��
��3����D���ɣ�C4H6O2��n�ķ�Ӧ����Ϊ ����G��J�ķ�Ӧ����Ϊ ��
��4��G�Ľṹ��ʽΪ ��
��5��A��ͬ���칹�����࣬��������������A������ͬ���칹���� �֣������������칹����
�ٺ������� �ں��Ȼ��� �ۺ�ȩ���� �ܺ�̼̼˫����
��1��̼̼˫����ȩ��������(�����ṹ���) (3��)
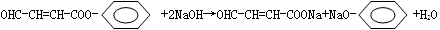 (2��)
(2��)
��2��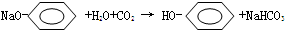 (2��)
(2��)
��3�����۷�Ӧ (2��) ��ȥ��Ӧ(2��)
��4��HOOCCHBrCHBrCOOH(2��)
��5��25 (2��)
��������
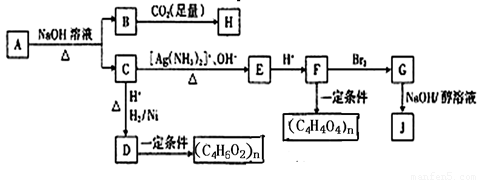
�����������A�������ܶ�����ͬ������H2��88����A����Է�������Ϊ176��H��FeCl3��Һ����ɫ����֪HΪ ��BΪ
��BΪ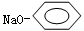 ��C����̼̼˫������Ϻϳ�·��ͼC����4��C������1��-CHO��1��-COONa��CΪOHC-CH=CH-COONa��AΪ
��C����̼̼˫������Ϻϳ�·��ͼC����4��C������1��-CHO��1��-COONa��CΪOHC-CH=CH-COONa��AΪ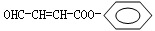 ��DΪHO-CH2-CH2-CH2-COOH��FΪHOOC-CH=CH-COOH��G�Ľṹ��ʽΪHOOCCHBrCHBrCOOH��JΪNaOOC-C��C-COONa��
��DΪHO-CH2-CH2-CH2-COOH��FΪHOOC-CH=CH-COOH��G�Ľṹ��ʽΪHOOCCHBrCHBrCOOH��JΪNaOOC-C��C-COONa��
��1��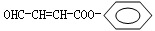 �к��еĹ�������̼̼˫����ȩ����������
�к��еĹ�������̼̼˫����ȩ����������
A��B+C�ķ�Ӧ����ʽΪ ��
��
��2��̼������ǿ�ڱ��ӣ�ǿ������Ƶ����ᣬ��������Һ��ͨ��������CO2����Һ����Ϊ���ɱ��Ӷ�����ǣ���Ӧ�Ļ�ѧ����ʽΪ��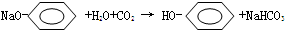 ��
��
��3��DΪHO-CH2-CH2-CH2-COOH����������(C4H6O2)n�����۷�Ӧ��NaOH/����Һ��±������ȥ��Ӧ��������
��4��G�Ľṹ��ʽΪHOOCCHBrCHBrCOOH��
��5��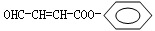 ���������ͬ���칹����25�֡�˼·������ϩ��ȡ��1���Ȼ���5�֣�Ȼ����5�ָ�ȡ��-CHO�ֱ��Ƕ����֡�
���������ͬ���칹����25�֡�˼·������ϩ��ȡ��1���Ȼ���5�֣�Ȼ����5�ָ�ȡ��-CHO�ֱ��Ƕ����֡�

���㣺�������л��ϳ�Ϊ�����������л������ŵ����ʼ��֮���ת�����л���Ӧ����ʽ��д����Ӧ���͡���������ͬ���칹�����д�����֪ʶ��






 OH+��n-1��H2O
OH+��n-1��H2O