��Ŀ����
��16�֣�A��B��C��D��E��F��G�����ֶ���������Ԫ�أ����ǵ�ԭ��������������������Ԫ�����ڱ���A��ԭ�Ӱ뾶��С��B��F��C��G�ֱ���ͬһ����Ԫ�أ�����DԪ�ص�������ɫ��ӦΪ��ɫ��GԪ�ص������������Ǵ�����������3/4����֪BԪ�ص��������������ڲ��������2����D��E��G���ߵ�����������ˮ�����������ܷ�Ӧ����ش�
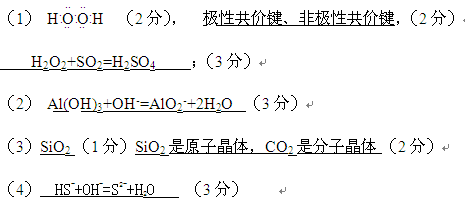
��1��A��C�γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽΪ �������ʺ�
��������GC2��Ӧ�Ļ�ѧ����ʽΪ
��2��D��E�����������ˮ���ﷴӦ�����ӷ���ʽΪ ��
��3��B��F����������� ���ѧʽ���ķе�ߣ�������
��4��A��C��G������γɼס������ָ�һ��˫ԭ�������ӣ�����18�����ӣ�����10�����ӣ�������ҷ�Ӧ�����ӷ���ʽΪ ��
��1��A��C�γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽΪ �������ʺ�
��������GC2��Ӧ�Ļ�ѧ����ʽΪ
��2��D��E�����������ˮ���ﷴӦ�����ӷ���ʽΪ ��
��3��B��F����������� ���ѧʽ���ķе�ߣ�������
��4��A��C��G������γɼס������ָ�һ��˫ԭ�������ӣ�����18�����ӣ�����10�����ӣ�������ҷ�Ӧ�����ӷ���ʽΪ ��
��Ԫ�����ڱ���A��ԭ�Ӱ뾶��С��A��H��B��F��C��G�ֱ���ͬһ����Ԫ�أ�����DԪ�ص�������ɫ��ӦΪ��ɫ,DΪNa��GԪ�ص������������Ǵ�����������3/4,GΪS,CΪO����֪BԪ�ص��������������ڲ��������2��,BΪC,FΪSi��D��E��G���ߵ�����������ˮ�����������ܷ�Ӧ,EΪAl��
A�DH��B�DC��C�DO��D�DNa��E�DAl��F�DSi��G�DS
�� ����H�DO���Թ��ۼ� O�DO�Ǽ��Թ��ۼ���H2O2��SO2=H2SO4;
��Al(OH)3��OH�D=AlO2�D��2H2O����SiO2 �е�ߣ�SiO2��ԭ�Ӿ��壬CO2�Ƿ��Ӿ��壻��HS�D��OH�D=S2�D��H2O.

��ϰ��ϵ�д�
 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
�����Ŀ
 ��H
��H ]
]
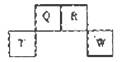
 ��W��R������������Ӧˮ��������ԣ�W>R
��W��R������������Ӧˮ��������ԣ�W>R