��Ŀ����
��֪A��B��֧�Թ���ʢ����Һ�й�����K����Ag����Mg2����Cl����OH����NO�������ӣ����Թ�A����Һ�е����̪��Һ�ʷۺ�ɫ����ش��������⣺
(1)�Թ�A����Һ�������������ӹ���________�֡�
(2)����ij�Թ��е���ϡ�����������������Թ�Ϊ______(�A����B��)��
(3)�����Թ�B����Һ�м�����ʵ�ҩƷ�����˺���Եõ���Ӧ�Ľ����ͽ���һ�����ʵ���Һ��������ҩƷ��__________(�ѧʽ)��
(4)�����Թ�A���Թ�B�е���Һ��һ������Ȼ�Ϲ��˺�������Һ�ɵõ�һ�ִ�������Ϲ����з�����Ӧ�����ӷ���ʽΪ___________________________________��_________________��
(5)���Թ�A���Թ�B�е���Һ���ɵ����ʵ���������������ɵ���Һ�������Թ�����Һ��Ϻ���ˣ�������Һ�и������ӵ����ʵ���֮��Ϊ________��
(6)�������Թ�A��Һ�е���������ɵ�̼��������Һ�У���������Ba(OH)2��Һ��������Ӧ�����ӷ���ʽΪ________________
(1)�Թ�A����Һ�������������ӹ���________�֡�
(2)����ij�Թ��е���ϡ�����������������Թ�Ϊ______(�A����B��)��
(3)�����Թ�B����Һ�м�����ʵ�ҩƷ�����˺���Եõ���Ӧ�Ľ����ͽ���һ�����ʵ���Һ��������ҩƷ��__________(�ѧʽ)��
(4)�����Թ�A���Թ�B�е���Һ��һ������Ȼ�Ϲ��˺�������Һ�ɵõ�һ�ִ�������Ϲ����з�����Ӧ�����ӷ���ʽΪ___________________________________��_________________��
(5)���Թ�A���Թ�B�е���Һ���ɵ����ʵ���������������ɵ���Һ�������Թ�����Һ��Ϻ���ˣ�������Һ�и������ӵ����ʵ���֮��Ϊ________��
(6)�������Թ�A��Һ�е���������ɵ�̼��������Һ�У���������Ba(OH)2��Һ��������Ӧ�����ӷ���ʽΪ________________
(1)3��(2)B��(3)Mg (4)Ag����Cl��===AgCl����Mg2����2OH��===Mg(OH)2��
(5)n(K��)��n(Mg2��)��n(NO)��4��1��6
(6)Ba2����2OH����2HCO===BaCO3����CO��2H2O
(5)n(K��)��n(Mg2��)��n(NO)��4��1��6
(6)Ba2����2OH����2HCO===BaCO3����CO��2H2O
��1�����Թ�A����Һ�е����̪��Һ�ʷۺ�ɫ��˵����Һ�Լ��ԡ�����Ag����Cl�����ܴ������棬����A�к��е���K����Cl����OH������A�к���Ag����Mg2����NO��
��2��Ag����Cl���ܲ����Ȼ�������������һ����B�Թܡ�
��3����Ӧ����Һ�н���һ�����ʣ����Ը��Լ���þ��
��4������AB�к��е����ӿ�֪�����ʱ��Ӧ�����ӷ���ʽΪAg����Cl��===AgCl����Mg2����2OH��===Mg(OH)2����
��5�������ǵ����ʵ�����ϣ����Ը��ݷ���ʽ��֪������þ�ǹ����ģ�����Ӧ����Һ�к�������غ�����þ��������Һ��n(K��)��n(Mg2��)��n(NO)��4��1��6��
��6����������������㣬��̼����غ�����������Ӧ����������̼�ᱵ��̼��غ�ˮ������ʽΪBa2����2OH����2HCO===BaCO3����CO��2H2O��
��2��Ag����Cl���ܲ����Ȼ�������������һ����B�Թܡ�
��3����Ӧ����Һ�н���һ�����ʣ����Ը��Լ���þ��
��4������AB�к��е����ӿ�֪�����ʱ��Ӧ�����ӷ���ʽΪAg����Cl��===AgCl����Mg2����2OH��===Mg(OH)2����
��5�������ǵ����ʵ�����ϣ����Ը��ݷ���ʽ��֪������þ�ǹ����ģ�����Ӧ����Һ�к�������غ�����þ��������Һ��n(K��)��n(Mg2��)��n(NO)��4��1��6��
��6����������������㣬��̼����غ�����������Ӧ����������̼�ᱵ��̼��غ�ˮ������ʽΪBa2����2OH����2HCO===BaCO3����CO��2H2O��

��ϰ��ϵ�д�
 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�
�����Ŀ
 ��SO42-��CO32-,���ֻ�������������Ӿ�����ͬ���Ҿ�������ˮ���ֽ����Ƿֱ����0.1 mol��L-1����Һ����������ʵ�飺
��SO42-��CO32-,���ֻ�������������Ӿ�����ͬ���Ҿ�������ˮ���ֽ����Ƿֱ����0.1 mol��L-1����Һ����������ʵ�飺 ��
��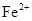 ��
�� ��
�� ��ϡ��Һ�У�����������
��ϡ��Һ�У����������� ���壬��ַ�Ӧ���ټ��������ϡ���ᣬ��ȫ��Ӧ��������Ŀû�б仯���ǣ�����
���壬��ַ�Ӧ���ټ��������ϡ���ᣬ��ȫ��Ӧ��������Ŀû�б仯���ǣ�����