��Ŀ����
��������ι�ҵ����Ҫԭ�ϣ���þʯ������ֽ�ķ�ӦʽΪ��2MgO?B2O3?H2O+2H2SO4��2H3BO3+2MgSO4��
��1��������Ӧ�У���������Ų�ʽ��ͬ�������� ��ԭ�Ӻ���������ֲ�ͬ�������ӵ�Ԫ�������� ��
��2����Ԫ����Ԫ�����ڱ��е�λ���� ����֪BF3���ӵĿռ乹��Ϊƽ���������Σ���BF3���� ���ӣ�����ԡ��Ǽ��ԡ�����
��3��MgԪ�ص�ԭ�ӽṹʾ��ͼΪ ��Mg��OH��2���Ա�Ca��OH��2 ���ǿ������������
��4������[H3BO3��B��OH��3]��һԪ���ᣬ����ˮ��Һ֮���Գ������Բ��DZ��������H+������������ˮ����ʱ����ˮ���������OH-��ϣ�������Һ��c��H+����c��OH-���������ӷ���ʽ��ʾ��������Ե�ԭ�� ��
��1��������Ӧ�У���������Ų�ʽ��ͬ��������
��2����Ԫ����Ԫ�����ڱ��е�λ����
��3��MgԪ�ص�ԭ�ӽṹʾ��ͼΪ
��4������[H3BO3��B��OH��3]��һԪ���ᣬ����ˮ��Һ֮���Գ������Բ��DZ��������H+������������ˮ����ʱ����ˮ���������OH-��ϣ�������Һ��c��H+����c��OH-���������ӷ���ʽ��ʾ��������Ե�ԭ��
��������1��þ���Ӻ������Ӻ�������Ų�ʽ��ͬ��ԭ�Ӻ����м����ܼ����м��ֲ�ͬ�����ĵ��ӣ�
��2��Bԭ�Ӻ�����2�����Ӳ㣬�������3�����ӣ����ݵ��Ӳ�������������ȷ�������ڱ��е�λ�ã�
������������غϵķ���Ϊ�Ǽ��Է��ӣ�����������IJ��غϵķ���Ϊ���Է��ӣ�
��3��þԭ�Ӻ�����12�����ӣ���3�����Ӳ㣬�������2�����ӣ�������Խǿ��Ԫ�����ļ���Խǿ��
��4��������ˮ���ã�B��OH��3+H20?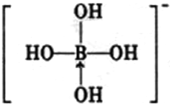 +H+��������Һ�����ԣ�
+H+��������Һ�����ԣ�
��2��Bԭ�Ӻ�����2�����Ӳ㣬�������3�����ӣ����ݵ��Ӳ�������������ȷ�������ڱ��е�λ�ã�
������������غϵķ���Ϊ�Ǽ��Է��ӣ�����������IJ��غϵķ���Ϊ���Է��ӣ�
��3��þԭ�Ӻ�����12�����ӣ���3�����Ӳ㣬�������2�����ӣ�������Խǿ��Ԫ�����ļ���Խǿ��
��4��������ˮ���ã�B��OH��3+H20?
 +H+��������Һ�����ԣ�
+H+��������Һ�����ԣ�����⣺��1��O 2-��Mg 2+���ⶼ��10���ӣ�����������Ų�ʽ��ͬ��ԭ�Ӻ����м����ܼ����м��ֲ�ͬ�����ĵ��ӣ���ԭ�Ӻ�����1S��2S��2P��3S��3P�����ܼ�������ԭ�Ӻ���������ֲ�ͬ�������ӵ�Ԫ���������ʴ�Ϊ��O 2-��Mg 2+����
��2��Bԭ�Ӻ�����5�����ӣ����������Ӳ㣬����������3������BԪ�ش��ڵڶ����ڵ�IIIA�壬BF3���ӵĿռ乹��Ϊƽ���������Σ�������������غϣ������ǷǼ��Է��ӣ��ʴ�Ϊ���ڶ����ڵ�IIIA�壻�Ǽ��ԣ�
��3��þԭ�Ӻ�����3�����Ӳ㣬�������3�����ӣ���ԭ�ӽṹʾ��ͼΪ��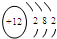 ��������Խǿ��Ԫ�����ļ���Խǿ���ƵĽ����Դ���þ�������������Ƶļ��Դ���������þ���ʴ�Ϊ��
��������Խǿ��Ԫ�����ļ���Խǿ���ƵĽ����Դ���þ�������������Ƶļ��Դ���������þ���ʴ�Ϊ��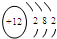 ������
������
��4��������ˮ����ʱ����ˮ���������OH-��ϣ�����[B��OH��4]-��������Һ��c��H+����c��OH-�������ӷ���ʽΪ��B��OH��3+H2O?H++[B��OH��4]-���ʴ�Ϊ��B��OH��3+H2O?H++[B��OH��4]-��
��2��Bԭ�Ӻ�����5�����ӣ����������Ӳ㣬����������3������BԪ�ش��ڵڶ����ڵ�IIIA�壬BF3���ӵĿռ乹��Ϊƽ���������Σ�������������غϣ������ǷǼ��Է��ӣ��ʴ�Ϊ���ڶ����ڵ�IIIA�壻�Ǽ��ԣ�
��3��þԭ�Ӻ�����3�����Ӳ㣬�������3�����ӣ���ԭ�ӽṹʾ��ͼΪ��
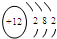 ��������Խǿ��Ԫ�����ļ���Խǿ���ƵĽ����Դ���þ�������������Ƶļ��Դ���������þ���ʴ�Ϊ��
��������Խǿ��Ԫ�����ļ���Խǿ���ƵĽ����Դ���þ�������������Ƶļ��Դ���������þ���ʴ�Ϊ��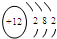 ������
��������4��������ˮ����ʱ����ˮ���������OH-��ϣ�����[B��OH��4]-��������Һ��c��H+����c��OH-�������ӷ���ʽΪ��B��OH��3+H2O?H++[B��OH��4]-���ʴ�Ϊ��B��OH��3+H2O?H++[B��OH��4]-��
�����������Ѷ��еȣ��ۺ���ǿ���漰��֪ʶ��࣬����Щ֪ʶ�㶼�����ʽṹ�е��ص㣬ƽʱӦ��ע������ܽᣮ

��ϰ��ϵ�д�
�����Ŀ
 2NH3��g��+
2NH3��g��+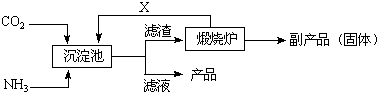
 5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
 NaC1O3+3H2��
NaC1O3+3H2�� HC1O+H++C1��
HC1O
HC1O+H++C1��
HC1O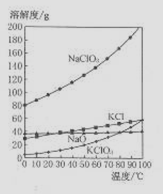
 5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��
5NaC1+NaC1O3+3H2O�����ڡ�5���NaOH��Һ��ͨ������C12��ƽ�ⳣ��K=1.09��1012������ʱC12������������Ҫ�� ������Һ���ȣ���Һ����Ҫ����Ũ�����¶ȵı仯����ͼ��ʾ��ͼ��A��B��C���α�ʾ�������� ��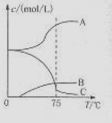
 NaC1O3+3H2��
NaC1O3+3H2�� HC1O+H++C1�� HC1O
HC1O+H++C1�� HC1O