��Ŀ����
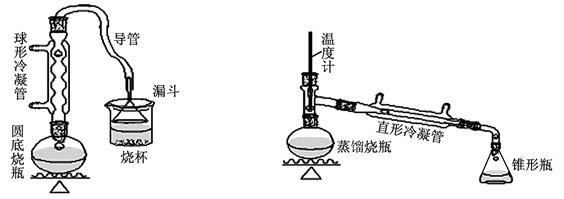
��9�֣�ij��ȤС��ͬѧ��ʵ�����ü���l��������ŨH2SO4���廯�ƻ����ķ������Ʊ�1���嶡�飬���������ͼ��ʾ��ʵ��װ�ã����еļг�����û�л�������
��ش��������⣺
��1��Aװ���У����ձ����Һ�浹��һ��©������Ŀ���� �� ��
��2���Ʊ������У������Ũ�������ȱ������ϡ�ͣ���Ŀ���� �� ��������ĸ��
a�����ٸ�����ϩ���ѵ��� �� b������Br2������ c��ˮ�Ƿ�Ӧ�Ĵ���
�� b������Br2������ c��ˮ�Ƿ�Ӧ�Ĵ���
��3����ͬѧ��ͨ����������Ǽ������ò������Ƿ� ���С���CH2CH2CH2CH3������ȷ�����������Ƿ���ڶ��ѣ�CH3CH2CH2CH2OCH2CH2CH2CH3���������۸�ͬѧ��Ƶļ��������Ƿ������Ϊʲô���� �� ��
���С���CH2CH2CH2CH3������ȷ�����������Ƿ���ڶ��ѣ�CH3CH2CH2CH2OCH2CH2CH2CH3���������۸�ͬѧ��Ƶļ��������Ƿ������Ϊʲô���� �� ��
��4��Ϊ�˽�һ���ᴿ1���嶡�飬��С��ͬѧ�������л�����й��������±���
����Bװ����ɴ��ᴿʵ��ʱ��ʵ����ҪѸ�������¶��� �� �ռ�������֡�

��ش��������⣺
��1��Aװ���У����ձ����Һ�浹��һ��©������Ŀ���� �� ��
��2���Ʊ������У������Ũ�������ȱ������ϡ�ͣ���Ŀ���� �� ��������ĸ��
a�����ٸ�����ϩ���ѵ���
 �� b������Br2������ c��ˮ�Ƿ�Ӧ�Ĵ���
�� b������Br2������ c��ˮ�Ƿ�Ӧ�Ĵ�����3����ͬѧ��ͨ����������Ǽ������ò������Ƿ�
 ���С���CH2CH2CH2CH3������ȷ�����������Ƿ���ڶ��ѣ�CH3CH2CH2CH2OCH2CH2CH2CH3���������۸�ͬѧ��Ƶļ��������Ƿ������Ϊʲô���� �� ��
���С���CH2CH2CH2CH3������ȷ�����������Ƿ���ڶ��ѣ�CH3CH2CH2CH2OCH2CH2CH2CH3���������۸�ͬѧ��Ƶļ��������Ƿ������Ϊʲô���� �� ����4��Ϊ�˽�һ���ᴿ1���嶡�飬��С��ͬѧ�������л�����й��������±���
| ���� | �۵㣯�� | �е㣯�� |
| 1������ | ��89.5 | 117.3 |
1���� ���� ���� | ��112.4 | 101.6 |
| ���� | ��95.3 | 142.4 |
| 1����ϩ | ��185.3 | ��6.5 |
����Bװ����ɴ��ᴿʵ��ʱ��ʵ����ҪѸ�������¶��� �� �ռ�������֡�
��9�֣�ÿС��2�֣�����С��3�֣�
��1���ȿ���ʹ���ճ�֣��ֿ��Է�ֹ����
��2��ab
��3��������������1���嶡��Ҳ���У�CH2CH2CH2CH3
��4��101.6��
��1���ȿ���ʹ���ճ�֣��ֿ��Է�ֹ����
��2��ab
��3��������������1���嶡��Ҳ���У�CH2CH2CH2CH3
��4��101.6��
��

��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ
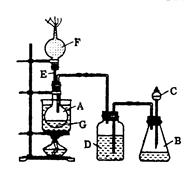
 __________��
__________��
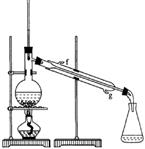
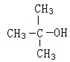
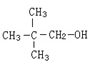
 �У��йػ�ѧ��������������˵������ȷ����
�У��йػ�ѧ��������������˵������ȷ����